Novel Non-Phosphorylative Pathway of Pentose Metabolism from Bacteria
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Comparison of Topological Clustering Within Protein Networks Using Edge
Comparison of topological clustering within protein networks using edge metrics that evaluate full sequence, full structure, and active site microenvironment similarity Janelle B. Leuthaeuser,1 Stacy T. Knutson,2 Kiran Kumar,2 Patricia C. Babbitt,3,4 and Jacquelyn S. Fetrow1,2* 1Department of Molecular Genetics and Genomics, Wake Forest University, Winston-Salem, North Carolina 27106 2Departments of Computer Science and Physics, Wake Forest University, Winston-Salem, North Carolina 27106 3Department of Bioengineering and Therapeutic Sciences, Institute for Quantitative Biosciences University of California San Francisco, San Francisco, California 94158 4Department of Pharmaceutical Chemistry, Institute for Quantitative Biosciences University of California San Francisco, San Francisco, California 94158 Received 10 April 2015; Accepted 10 June 2015 DOI: 10.1002/pro.2724 Published online 12 June 2015 proteinscience.org Abstract: The development of accurate protein function annotation methods has emerged as a major unsolved biological problem. Protein similarity networks, one approach to function annota- tion via annotation transfer, group proteins into similarity-based clusters. An underlying assump- tion is that the edge metric used to identify such clusters correlates with functional information. In this contribution, this assumption is evaluated by observing topologies in similarity networks using three different edge metrics: sequence (BLAST), structure (TM-Align), and active site similarity (active site profiling, implemented in DASP). Network topologies for four well-studied protein superfamilies (enolase, peroxiredoxin (Prx), glutathione transferase (GST), and crotonase) were compared with curated functional hierarchies and structure. As expected, network topology differs, depending on edge metric; comparison of topologies provides valuable information on structure/ function relationships. Subnetworks based on active site similarity correlate with known functional hierarchies at a single edge threshold more often than sequence- or structure-based networks. -

Sulfite Dehydrogenases in Organotrophic Bacteria : Enzymes
Sulfite dehydrogenases in organotrophic bacteria: enzymes, genes and regulation. Dissertation zur Erlangung des akademischen Grades des Doktors der Naturwissenschaften (Dr. rer. nat.) an der Universität Konstanz Fachbereich Biologie vorgelegt von Sabine Lehmann Tag der mündlichen Prüfung: 10. April 2013 1. Referent: Prof. Dr. Bernhard Schink 2. Referent: Prof. Dr. Andrew W. B. Johnston So eine Arbeit wird eigentlich nie fertig, man muss sie für fertig erklären, wenn man nach Zeit und Umständen das möglichste getan hat. (Johann Wolfgang von Goethe, Italienische Reise, 1787) DANKSAGUNG An dieser Stelle möchte ich mich herzlich bei folgenden Personen bedanken: . Prof. Dr. Alasdair M. Cook (Universität Konstanz, Deutschland), der mir dieses Thema und seine Laboratorien zur Verfügung stellte, . Prof. Dr. Bernhard Schink (Universität Konstanz, Deutschland), für seine spontane und engagierte Übernahme der Betreuung, . Prof. Dr. Andrew W. B. Johnston (University of East Anglia, UK), für seine herzliche und bereitwillige Aufnahme in seiner Arbeitsgruppe, seiner engagierten Unter- stützung, sowie für die Übernahme des Koreferates, . Prof. Dr. Frithjof C. Küpper (University of Aberdeen, UK), für seine große Hilfsbereitschaft bei der vorliegenden Arbeit und geplanter Manuskripte, als auch für die mentale Unterstützung während der letzten Jahre! Desweiteren möchte ich herzlichst Dr. David Schleheck für die Übernahme des Koreferates der mündlichen Prüfung sowie Prof. Dr. Alexander Bürkle, für die Übernahme des Prüfungsvorsitzes sowie für seine vielen hilfreichen Ratschläge danken! Ein herzliches Dankeschön geht an alle beteiligten Arbeitsgruppen der Universität Konstanz, der UEA und des SAMS, ganz besonders möchte ich dabei folgenden Personen danken: . Dr. David Schleheck und Karin Denger, für die kritische Durchsicht dieser Arbeit, der durch und durch sehr engagierten Hilfsbereitschaft bei Problemen, den zahlreichen wissenschaftlichen Diskussionen und für die aufbauenden Worte, . -

Integrated Molecular Analysis of Sugar Metabolism of Sulfolobus Solfataricus
Integrated molecular analysis of Sugar Metabolism of Sulfolobus solfataricus Stan J.J. Brouns Integrated molecular analysis of sugar metabolism of Sulfolobus solfataricus Stan J.J. Brouns Promotoren prof. dr. Willem M. de Vos hoogleraar in de Microbiologie Wageningen Universiteit prof. dr. John van der Oost persoonlijk hoogleraar Microbiologie en Biochemie Wageningen Universiteit Leden van de prof. dr. Ton J.W.G. Visser promotie bijzonder hoogleraar Microspectroscopie in de Biochemie commissie Wageningen Universiteit prof. dr. Arnold J.M. Driessen hoogleraar Moleculaire Microbiologie Rijksuniversiteit Groningen dr. Loren L. Looger Howard Hughes Medical Institute Ashburn (VA), Verenigde Staten dr. Thijs Kaper Genencor International Palo Alto (CA), Verenigde Staten Dit onderzoek is uitgevoerd binnen de onderzoekschool VLAG Integrated molecular analysis of sugar metabolism of Sulfolobus solfataricus Stan J.J. Brouns Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, prof. dr. M.J. Kropff, in het openbaar te verdedigen op dinsdag 2 oktober 2007 des namiddags te half twee in de Aula Cover Boiling water: the habitat of a hyperthermophile (photo: S.J.J. Brouns) Printing Gildeprint (Enschede) Sponsoring Hellma Benelux, bioMérieux Brouns, S.J.J. - Integrated molecular analysis of sugar metabolism of Sulfolobus solfataricus in Dutch Geïntegreerde moleculaire analyse van het suikermetabolisme van Sulfolobus solfataricus PhD Thesis Wageningen University, Wageningen, Netherlands (2007) 176 p. - with summary in Dutch ISBN 978-90-8504-713-1 Voor mijn ouders en Marloes DANkwooRD Met trots ligt ligt hier nu een boekje. Natuurlijk kon het niet totstandkomen zonder de hulp van anderen. In dit stukje wil ik die mensen bedanken. -

Functional Assignments in the Enolase Superfamily: Investigations of Two Divergent Groups of D-Galacturonate Dehydratases and Galactarate Dehydratase-Iii
FUNCTIONAL ASSIGNMENTS IN THE ENOLASE SUPERFAMILY: INVESTIGATIONS OF TWO DIVERGENT GROUPS OF D-GALACTURONATE DEHYDRATASES AND GALACTARATE DEHYDRATASE-III BY FIONA PATRICIA GRONINGER-POE i DISSERTATION Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Biochemistry in the Graduate College of the University of Illinois at Urbana-Champaign, 2014 Urbana, Illinois Doctoral Committee: Professor John A. Gerlt, Chair Professor John E. Cronan, Jr. Associate Professor Rutilo Fratti Associate Professor Raven H. Huang ABSTRACT More than a decade after the genomic age, full genome sequencing is cost-effective and fast, allowing for the deposit of an ever increasing number of DNA sequences. New fields have arisen from this availability of genomic information, and the way we think about biochemistry and enzymology has been transformed. Unfortunately, there is no robust method for accurately determining the functions of enzymes encoded by these sequences that matches the speed in which genomes are deposited into public databases. Functional assignment of enzymes remains of utmost importance in understanding microbial metabolism and has applications in agriculture by examining bacterial plant pathogen metabolism and additionally in human health by providing metabolic context to the human gut microbiome. To aid in the functional identification of proteins, enzymes can be grouped into superfamilies which share common structural motifs as well as mechanistic features. To this end, the enolase superfamily is an excellent model system for functional assignment because more than half of the members still lack functional identification. Structurally, these enzymes contain substrate specificity residues in the N-terminal capping domain and catalytic residues in the C-terminal barrel domain. -

Metatranscriptomic Analysis of Community Structure And
School of Environmental Sciences Metatranscriptomic analysis of community structure and metabolism of the rhizosphere microbiome by Thomas Richard Turner Submitted in partial fulfilment of the requirement for the degree of Doctor of Philosophy, September 2013 This copy of the thesis has been supplied on condition that anyone who consults it is understood to recognise that its copyright rests with the author and that use of any information derived there from must be in accordance with current UK Copyright Law. In addition, any quotation or extract must include full attribution. i Declaration I declare that this is an account of my own research and has not been submitted for a degree at any other university. The use of material from other sources has been properly and fully acknowledged, where appropriate. Thomas Richard Turner ii Acknowledgements I would like to thank my supervisors, Phil Poole and Alastair Grant, for their continued support and guidance over the past four years. I’m grateful to all members of my lab, both past and present, for advice and friendship. Graham Hood, I don’t know how we put up with each other, but I don’t think I could have done this without you. Cheers Salt! KK, thank you for all your help in the lab, and for Uma’s biryanis! Andrzej Tkatcz, thanks for the useful discussions about our projects. Alison East, thank you for all your support, particularly ensuring Graham and I did not kill each other. I’m grateful to Allan Downie and Colin Murrell for advice. For sequencing support, I’d like to thank TGAC, particularly Darren Heavens, Sophie Janacek, Kirsten McKlay and Melanie Febrer, as well as John Walshaw, Mark Alston and David Swarbreck for bioinformatic support. -

Anti-Inflammatory Role of Curcumin in LPS Treated A549 Cells at Global Proteome Level and on Mycobacterial Infection
Anti-inflammatory Role of Curcumin in LPS Treated A549 cells at Global Proteome level and on Mycobacterial infection. Suchita Singh1,+, Rakesh Arya2,3,+, Rhishikesh R Bargaje1, Mrinal Kumar Das2,4, Subia Akram2, Hossain Md. Faruquee2,5, Rajendra Kumar Behera3, Ranjan Kumar Nanda2,*, Anurag Agrawal1 1Center of Excellence for Translational Research in Asthma and Lung Disease, CSIR- Institute of Genomics and Integrative Biology, New Delhi, 110025, India. 2Translational Health Group, International Centre for Genetic Engineering and Biotechnology, New Delhi, 110067, India. 3School of Life Sciences, Sambalpur University, Jyoti Vihar, Sambalpur, Orissa, 768019, India. 4Department of Respiratory Sciences, #211, Maurice Shock Building, University of Leicester, LE1 9HN 5Department of Biotechnology and Genetic Engineering, Islamic University, Kushtia- 7003, Bangladesh. +Contributed equally for this work. S-1 70 G1 S 60 G2/M 50 40 30 % of cells 20 10 0 CURI LPSI LPSCUR Figure S1: Effect of curcumin and/or LPS treatment on A549 cell viability A549 cells were treated with curcumin (10 µM) and/or LPS or 1 µg/ml for the indicated times and after fixation were stained with propidium iodide and Annexin V-FITC. The DNA contents were determined by flow cytometry to calculate percentage of cells present in each phase of the cell cycle (G1, S and G2/M) using Flowing analysis software. S-2 Figure S2: Total proteins identified in all the three experiments and their distribution betwee curcumin and/or LPS treated conditions. The proteins showing differential expressions (log2 fold change≥2) in these experiments were presented in the venn diagram and certain number of proteins are common in all three experiments. -

Noelia Díaz Blanco
Effects of environmental factors on the gonadal transcriptome of European sea bass (Dicentrarchus labrax), juvenile growth and sex ratios Noelia Díaz Blanco Ph.D. thesis 2014 Submitted in partial fulfillment of the requirements for the Ph.D. degree from the Universitat Pompeu Fabra (UPF). This work has been carried out at the Group of Biology of Reproduction (GBR), at the Department of Renewable Marine Resources of the Institute of Marine Sciences (ICM-CSIC). Thesis supervisor: Dr. Francesc Piferrer Professor d’Investigació Institut de Ciències del Mar (ICM-CSIC) i ii A mis padres A Xavi iii iv Acknowledgements This thesis has been made possible by the support of many people who in one way or another, many times unknowingly, gave me the strength to overcome this "long and winding road". First of all, I would like to thank my supervisor, Dr. Francesc Piferrer, for his patience, guidance and wise advice throughout all this Ph.D. experience. But above all, for the trust he placed on me almost seven years ago when he offered me the opportunity to be part of his team. Thanks also for teaching me how to question always everything, for sharing with me your enthusiasm for science and for giving me the opportunity of learning from you by participating in many projects, collaborations and scientific meetings. I am also thankful to my colleagues (former and present Group of Biology of Reproduction members) for your support and encouragement throughout this journey. To the “exGBRs”, thanks for helping me with my first steps into this world. Working as an undergrad with you Dr. -

Prioritization of Metabolic Genes As Novel Therapeutic Targets in Estrogen-Receptor Negative Breast Tumors Using Multi-Omics Data and Text Mining
www.oncotarget.com Oncotarget, 2019, Vol. 10, (No. 39), pp: 3894-3909 Research Paper Prioritization of metabolic genes as novel therapeutic targets in estrogen-receptor negative breast tumors using multi-omics data and text mining Dinesh Kumar Barupal1,*, Bei Gao1,*, Jan Budczies2, Brett S. Phinney4, Bertrand Perroud4, Carsten Denkert2,3 and Oliver Fiehn1 1West Coast Metabolomics Center, University of California, Davis, CA, USA 2Institute of Pathology, Charité University Hospital, Berlin, Germany 3German Institute of Pathology, Philipps-University Marburg, Marburg, Germany 4UC Davis Genome Center, University of California, Davis, CA, USA *Co-first authors and contributed equally to this work Correspondence to: Oliver Fiehn, email: [email protected] Keywords: set-enrichment; ChemRICH; multi-omics; metabolic networks; candidate gene prioritization Received: March 12, 2019 Accepted: May 13, 2019 Published: June 11, 2019 Copyright: Barupal et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License 3.0 (CC BY 3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. ABSTRACT Estrogen-receptor negative (ERneg) breast cancer is an aggressive breast cancer subtype in the need for new therapeutic options. We have analyzed metabolomics, proteomics and transcriptomics data for a cohort of 276 breast tumors (MetaCancer study) and nine public transcriptomics datasets using univariate statistics, meta- analysis, Reactome pathway analysis, biochemical network mapping and text mining of metabolic genes. In the MetaCancer cohort, a total of 29% metabolites, 21% proteins and 33% transcripts were significantly different (raw p <0.05) between ERneg and ERpos breast tumors. -

Dissimilation of Cysteate Via 3-Sulfolactate Sulfo-Lyase and a Sulfate Exporter in Paracoccus Pantotrophus NKNCYSA
Microbiology (2005), 151, 737–747 DOI 10.1099/mic.0.27548-0 Dissimilation of cysteate via 3-sulfolactate sulfo-lyase and a sulfate exporter in Paracoccus pantotrophus NKNCYSA Ulrike Rein,1 Ronnie Gueta,1 Karin Denger,1 Ju¨rgen Ruff,1 Klaus Hollemeyer2 and Alasdair M. Cook1 Correspondence 1Department of Biology, The University, D-78457 Konstanz, Germany Alasdair Cook 2Institute of Biochemical Engineering, Saarland University, Box 50 11 50, D-66041 [email protected] Saarbru¨cken, Germany Paracoccus pantotrophus NKNCYSA utilizes (R)-cysteate (2-amino-3-sulfopropionate) as a sole source of carbon and energy for growth, with either nitrate or molecular oxygen as terminal electron acceptor, and the specific utilization rate of cysteate is about 2 mkat (kg protein)”1. The initial degradative reaction is catalysed by an (R)-cysteate : 2-oxoglutarate aminotransferase, which yields 3-sulfopyruvate. The latter was reduced to 3-sulfolactate by an NAD-linked sulfolactate dehydrogenase [3?3 mkat (kg protein)”1]. The inducible desulfonation reaction was not detected initially in cell extracts. However, a strongly induced protein with subunits of 8 kDa (a) and 42 kDa (b) was found and purified. The corresponding genes had similarities to those encoding altronate dehydratases, which often require iron for activity. The purified enzyme could then be shown to convert 3-sulfolactate to sulfite and pyruvate and it was termed sulfolactate sulfo-lyase (Suy). A high level of sulfite dehydrogenase was also induced during growth with cysteate, and the organism excreted sulfate. A putative regulator, OrfR, was encoded upstream of suyAB on the reverse strand. Downstream of suyAB was suyZ, which was cotranscribed with suyB. -

Fine Mapping and Expression of Candidate Genes Within the Chromosome 10 QTL Region of the High and Low Alcohol Drinking Rats
View metadata, citation and similar papers at core.ac.uk brought to you by CORE HHS Public Access provided by IUPUIScholarWorks Author manuscript Author ManuscriptAuthor Manuscript Author Alcohol Manuscript Author . Author manuscript; Manuscript Author available in PMC 2017 July 18. Published in final edited form as: Alcohol. 2010 September ; 44(6): 477–485. doi:10.1016/j.alcohol.2010.06.004. Fine mapping and expression of candidate genes within the Chromosome 10 QTL region of the High and Low Alcohol Drinking Rats Paula J. Bice1, Tiebing Liang1, Lili Zhang1, Tamara J. Graves1, Lucinda G. Carr1, Dongbing Lai2, Mark W. Kimpel3, and Tatiana Foroud2 1Department of Medicine, Indiana University School of Medicine, Indianapolis, Indiana 46202, USA 2Department of Medical and Molecular Genetics, Indiana University School of Medicine, Indianapolis, IN 46202, USA 3Department of Psychiatry, Indiana University School of Medicine, Indianapolis, IN 46202, USA Abstract The high and low alcohol-drinking (HAD and LAD) rats were selectively bred for differences in alcohol intake. The HAD/LAD rats originated from the N/Nih heterogeneous stock (HS) developed from intercrossing 8 inbred rat strains. The HAD × LAD F2 were genotyped, and a powerful analytical approach, utilizing ancestral recombination as well as F2 recombination, was used to narrow a QTL for alcohol drinking to a 2 cM region on distal chromosome 10 that was in common in the HAD1/LAD1 and HAD2/LAD2 analyses. Quantitative real time PCR (qRT-PCR) was used to examine mRNA expression of 6 candidate genes (Crebbp, Trap1, Gnptg, Clcn7, Fahd1, and Mapk8ip3) located within the narrowed QTL region in the HAD1/LAD1 rats. -

Computational Analyses of Small Molecules Activity from Phenotypic Screens
Computational analyses of small molecules activity from phenotypic screens Azedine Zoufir Hughes Hall This dissertation is submitted for the degree of Doctor of Philosophy July 2018 Declaration This thesis is submitted as the result of my own work and includes nothing which is the outcome of work done in collaboration except where specifically indicated in the text. It is not substantially the same as any that I have submitted, or, is being concurrently submitted for a degree or diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the preface and specified in the text. I further state that no substantial part of my dissertation has already been submitted, or, is being concurrently submitted for any such degree, diploma or other qualification at the University of Cambridge or any other University or similar institution except as declared in the Preface and specified in the text. This dissertation does not exceed the word limit of 60,000 words. Azedine Zoufir July 2018 Summary Title: Computational analyses of small molecules activity from phenotypic screens Author: Azedine Zoufir Drug discovery is no longer relying on the one gene-one disease paradigm nor on target-based screening alone to discover new drugs. Phenotypic-based screening is regaining momentum to discover new compounds since those assays provide an environment closer to the physiological state of the disease and allow to better anticipate off-target effects and other factors that can limit the efficacy of the drugs. However, uncovering the mechanism of action of the compounds active in those assays relies on in vitro techniques that are expensive and time- consuming. -
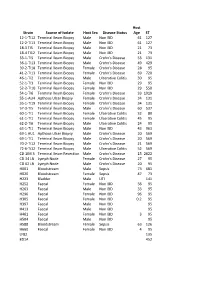
Strain Source of Isolate Host Sex Disease Status Host Age ST
Host Strain Source of Isolate Host Sex Disease Status Age ST 12-1-TI12 Terminal Ileum Biopsy Male Non IBD 61 127 12-2-TI13 Terminal Ileum Biopsy Male Non IBD 61 127 18-3 TI5 Terminal Ileum Biopsy Male Non IBD 21 73 18-4 TI12 Terminal Ileum Biopsy Male Non IBD 21 73 33-1-TI5 Terminal Ileum Biopsy Male Crohn's Disease 53 131 36-1-TI13 Terminal Ileum Biopsy Male Crohn's Disease 49 429 39-2-TI18 Terminal Ileum Biopsy Female Crohn's Disease 28 95 41-2-TI13 Terminal Ileum Biopsy Female Crohn's Disease 69 720 46-1-TI2 Terminal Ileum Biopsy Male Ulcerative Colitis 30 95 52-1-TI3 Terminal Ileum Biopsy Female Non IBD 29 95 52-2-TI10 Terminal Ileum Biopsy Female Non IBD 29 550 54-1-TI6 Terminal Ileum Biopsy Female Crohn's Disease 33 1919 55-1-AU4 Apthous Ulcer Biopsy Female Crohn's Disease 34 131 55-1-TI19 Terminal Ileum Biopsy Female Crohn's Disease 34 131 57-3-TI5 Terminal Ileum Biopsy Male Crohn's Disease 60 537 60-1-TI1 Terminal Ileum Biopsy Female Ulcerative Colitis 32 80 61-1-TI1 Terminal Ileum Biopsy Female Ulcerative Colitis 45 95 62-2-TI6 Terminal Ileum Biopsy Male Ulcerative Colitis 24 95 63-1-TI1 Terminal Ileum Biopsy Male Non IBD 43 963 69-1 AU1 Apthous Ulcer Biopsy Male Crohn's Disease 20 569 69-1-TI1 Terminal Ileum Biopsy Male Crohn's Disease 20 569 70-2-TI12 Terminal Ileum Biopsy Male Crohn's Disease 21 569 72-6-Ti12 Terminal Ileum Biopsy Male Ulcerative Colitis 52 569 CD 1IM 3 Terminal Ileum Resection Male Crohn's Disease 15 2622 CD 34 LN Lymph Node Female Crohn's Disease 27 95 CD 62 LN Lymph Node Male Crohn's Disease 20 95 H001