Laboratory Activities Biomedik I
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cell Biology and Fundamentals in Histology - En-Cours-2018-Liepr1004 Liepr1004 Cell Biology and Fundamentals in 2018 Histology
Université catholique de Louvain - Cell biology and fundamentals in histology - en-cours-2018-liepr1004 liepr1004 Cell biology and fundamentals in 2018 histology 5 credits 45.0 h Q2 Teacher(s) Behets Wydemans Catherine ;Henriet Patrick ; Language : French Place of the course Louvain-la-Neuve Main themes The major themes are : - Characteristics common to all living species - The human cell, its functioning and division - Classical, evolutive and molecular genetics - Cellular bases in sexual reproduction - The differents cell types and their organisation in tissues - The major steps in human embryonic development Aims By the end of the module, students should understand the bases of unicity and diversity in the living world. They will know the structure and functioning of human cell and genome as well as the mechanisms of 1 cell division and embryonic development. Moreover, they will know the structure of the major types of human tissues. - - - - The contribution of this Teaching Unit to the development and command of the skills and learning outcomes of the programme(s) can be accessed at the end of this sheet, in the section entitled “Programmes/courses offering this Teaching Unit”. Content (auteurs - titulaires actuels) : P. Henriet and Ph. van den Bosch de Aguilar 1. UNICITY IN THE LIVING WORLD 2. THE HUMAN CELL 3. DIVERSITY IN THE LIVING WORLD 4. MOLECULAR GENETICS 5. CELL DIVISION 6. GAMETOGENESIS AND FERTILIZATION 7. INTRODUCTION TO HUMAN EMBRYOLOGY Histology 1. EPITHELIAL TISSUE 2. CONNECTIVE TISSUE 3. BLOOD TISSUE 4. MUSCLE TISSUE -

Immune Response and Histology of Humoral Rejection in Kidney
Document downloaded from http://www.elsevier.es, day 23/05/2017. This copy is for personal use. Any transmission of this document by any media or format is strictly prohibited. n e f r o l o g i a 2 0 1 6;3 6(4):354–367 Revista de la Sociedad Española de Nefrología www.revistanefrologia.com Review Immune response and histology of humoral rejection in kidney transplantation a,∗ a b a Miguel González-Molina , Pedro Ruiz-Esteban , Abelardo Caballero , Dolores Burgos , a c a a Mercedes Cabello , Miriam Leon , Laura Fuentes , Domingo Hernandez a Nephrology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain b Immunology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain c Pathology Department, Regional University Hospital of Malaga, Malaga University, IBIMA, REDINREN RD12/0021/0015, Malaga, Spain a r t i c l e i n f o a b s t r a c t Article history: The adaptive immune response forms the basis of allograft rejection. Its weapons are direct Received 4 June 2015 cellular cytotoxicity, identified from the beginning of organ transplantation, and/or anti- Accepted 26 March 2016 bodies, limited to hyperacute rejection by preformed antibodies and not as an allogenic Available online 3 June 2016 response. This resulted in allogenic response being thought for decades to have just a cellu- lar origin. But the experimental studies by Gorer demonstrating tissue damage in allografts Keywords: due to antibodies secreted by B lymphocytes activated against polymorphic molecules were Immune response disregarded. -

Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses
The Journal of Neuroscience October 1986, 6(10): 3079-3085 Polyribosomes Associated with Synaptic Specializations on Axon Initial Segments: Localization of Protein-Synthetic Machinery at Inhibitory Synapses Oswald Steward* and Charles E. Ribak-f *Departments of Neurosurgery and Physiology, University of Virginia School of Medicine, Charlottesville, Virginia 22908, and TDepartment of Anatomy and Neurobiology, University of California at Irvine, Irvine, California 92717 Previous studies have revealed a selective association between report that showed some axonal ribosomes grouped at points polyribosomes and axospinous synapses in a variety of brain of branching and beneath synaptic contacts (Jones and Powell, regions. The present study evaluates whether polyribosomes are 1969). Therefore, they were usually considered, only in passing, also associated with the symmetrical and presumably inhibitory as exceptions to the rule. Thus, it has come to be accepted as a synaptic connections found on the initial segment of axons of central tenet that essentially all of the protein constituents of some neurons in the CNS. The initial segments of pyramidal the neuron, including those required for synaptic specializations, neurons in the sensorimotor cortex of the monkey and of granule were synthesized in the cell body, and somehow selectively cells in the hippocampus of the rat were examined. The initial transported to the sites at which they would be assembled (for segments of these cell types are contacted by GABAergic ter- a review, see Grafstein and Forman, 1980). minals that form symmetrical synaptic connections. In the pres- Over the past few years, a series of observations has given ent study, these initial segments were found to contain polyri- rise to an hypothesis that challenges the prevailing view that bosomes that tended to be selectively localized beneath the virtually all neuronal proteins are produced in the cell body and synaptic specializations. -

Satellitosis, a Crosstalk Between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour
cancers Review Satellitosis, a Crosstalk between Neurons, Vascular Structures and Neoplastic Cells in Brain Tumours; Early Manifestation of Invasive Behaviour Prospero Civita 1,2,* , Ortenzi Valerio 3 , Antonio Giuseppe Naccarato 3 , Mark Gumbleton 2 and Geoffrey J. Pilkington 1,2,4,* 1 Brain Tumour Research Centre, Institute of Biological and Biomedical Sciences (IBBS), School of Pharmacy and Biomedical Sciences, University of Portsmouth, Portsmouth PO1 2DT, UK 2 School of Pharmacy and Pharmaceutical Sciences, College of Biomedical and Life Sciences, Cardiff University, Cardiff CF10 3NB, UK; gumbleton@cardiff.ac.uk 3 Department of Translational Research and New Technologies in Medicine and Surgery, Pisa University Hospital, 56100 Pisa, Italy; [email protected] (O.V.); [email protected] (A.G.N.) 4 Division of Neuroscience, Department of Basic and Clinical Neuroscience, Institute of Psychiatry & Neurology, King’s College London, London SE5 9RX, UK * Correspondence: CivitaP@cardiff.ac.uk (P.C.); geoff[email protected] (G.J.P.) Received: 9 November 2020; Accepted: 4 December 2020; Published: 11 December 2020 Simple Summary: This article reviews the concept of cellular satellitosis as originally described histologically by Santiago Ramón y Cajal in 1899 and Hans Joachim Scherer, more specifically in the context of glioblastoma invasiveness, during the early part of the 20th century. With the advent of new and emerging molecular technologies in the 21st century, the significance of both vascular and neuronal satellitosis by neoplastic cells offers intriguing possibilities into further clarifying the development, pathobiology and therapy of malignant glioma through closer investigation into the nature of these histological hallmarks. -

Astrocyte Cell Culture Preparation of Flasks: 1
Astrocyte Cell Culture Preparation of flasks: 1. Coat T75 flask(s) with 1 mg/ml of PureCol (Collagen) overnight 2. Remove solution, rinse flasks with sterile ddH20, set the flasks upright and allow to dry in culture hood for 2 hr Dissection: 1. Dissect P1-P3 pups: Remove brainstem, cerebellum and diencephalons in cold dissection buffer. Peel off meninges and transfer cortex to a 50 ml tube on ice, which contains 20 ml of cold dissection buffer. (Dissect 2 pups for 2 x 106 cells/flask). 2. Carefully pour tissue into a 10 cm dish and gently mince tissue with sterile scissors or razor blade. 3. Transfer tissue to back to 50 ml tube and add 5 ml 1X trypsin and 50 uL DNAse for 25 min at 37ºC. Swirl tube every 5 min. 4. Wash the cortices with Glial Medium twice. 5. Dissociate the tissue by gently triturating the cortices through a 5 ml or 2 ml pipette, followed by a fire-polished Pasteur pipette (3 X 3 triturations). Each time fill pipette with dissociated cells and transfer supernatant to a fresh tube. 6. Dilute cell suspension to 10 ml of Glial Medium, and pass through a 40 uM cell strainer. 7. Spin down the cells at 1700 rpm for 5 min. 8. Re-suspend the cells with 10 ml of Glial Medium, and count. 9. Seed 2 x 106 cells/flask in 15 ml Glial medium. ****(2.0 x 106 cells/flask = 1.33 x 105 cells/ml = 2.67 x 104 cells/cm2)***** 10. Change the medium each of the next two days by aspirating the medium, and then adding back 15 ml of fresh Glial Medium. -
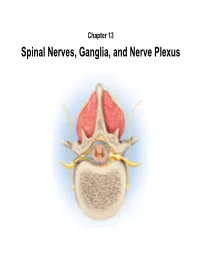
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

The Peripheral Nervous System
The Peripheral Nervous System Dr. Ali Ebneshahidi Peripheral Nervous System (PNS) – Consists of 12 pairs of cranial nerves and 31 pairs of spinal nerves. – Serves as a critical link between the body and the central nervous system. – peripheral nerves contain an outermost layer of fibrous connective tissue called epineurium which surrounds a thinner layer of fibrous connective tissue called perineurium (surrounds the bundles of nerve or fascicles). Individual nerve fibers within the nerve are surrounded by loose connective tissue called endoneurium. Cranial Nerves Cranial nerves are direct extensions of the brain. Only Nerve I (olfactory) originates from the cerebrum, the remaining 11 pairs originate from the brain stem. Nerve I (Olfactory)- for the sense of smell (sensory). Nerve II (Optic)- for the sense of vision (sensory). Nerve III (Oculomotor)- for controlling muscles and accessory structures of the eyes ( primarily motor). Nerve IV (Trochlear)- for controlling muscles of the eyes (primarily motor). Nerve V (Trigeminal)- for controlling muscles of the eyes, upper and lower jaws and tear glands (mixed). Nerve VI (Abducens)- for controlling muscles that move the eye (primarily motor). Nerve VII (Facial) – for the sense of taste and controlling facial muscles, tear glands and salivary glands (mixed). Nerve VIII (Vestibulocochlear)- for the senses of hearing and equilibrium (sensory). Nerve IX (Glossopharyngeal)- for controlling muscles in the pharynx and to control salivary glands (mixed). Nerve X (Vagus)- for controlling muscles used in speech, swallowing, and the digestive tract, and controls cardiac and smooth muscles (mixed). Nerve XI (Accessory)- for controlling muscles of soft palate, pharynx and larynx (primarily motor). Nerve XII (Hypoglossal) for controlling muscles that move the tongue ( primarily motor). -
PN1 (Midha) Microanatomy of Peripheral Nerves-Part1.Pdf
Peripheral Nerve Basics • Consist of processes of cell bodies found in the DRG, anterior horn, and autonomic ganglia • Organized by several distinct connective tissue layers – the epineurium, perineurium, and endoneurium • Vascular supply provided by the vasa nervorum Peripheral Nerve Basics • Neuronal processes bound into fascicles by perineurium • Fascicles bound into nerves by epineurium • Endoneurium is a division of the perineurium which form thin layers of connective tissue surrounding neuronal fibers in a fascicle Sural nerve in cross-section Epineurium • Loose areolar tissue with sparse, longitudinally-oriented collagen fibers • Some elastic fibers where epineurium abuts perineurium • Able to accommodate a significant amount of nerve stretching and movement • Increases in thickness where nerves cross joints • Constitutes an increasing proportion of nerves as they increase in size • Epineurial fat helps cushion nerves from compressive injury • Decreased epineurial fat found in patients with diabetes Perineurium • Cellular component composed of laminated fibroblasts of up to 15 layers in thickness which are bounded by a basal lamina • Semi-permeable: inner lamellae have tight junctions, providing a barrier to intercellular transport of macromolecules – Tight junctions can be loosened with topical anaesthetics and with osmotic change Perineurium • Exhibits a slightly positive internal pressure – Fascicular contents herniate upon perineurial injury • Under tension longitudinally – Nerve segment shortens upon transection – may complicate surgical repair as nerve can be stretched only approximately 10% before being inhibited by collagen Endoneurium • Intrafascicular connective tissue consisting of a collagenous matrix in the interstitial space • Develops into partitions of dense connective tissue between diverging fascicles and eventually becomes perineurium when the fascicles separate • Collagen fibers are longitudinally-oriented and run along nerve fibers and capillaries. -

Was Not Reached, However, Even After Six to Sevenhours. A
PROTEIN SYNTHESIS IN THE ISOLATED GIANT AXON OF THE SQUID* BY A. GIUDITTA,t W.-D. DETTBARN,t AND MIROSLAv BRZIN§ MARINE BIOLOGICAL LABORATORY, WOODS HOLE, MASSACHUSETTS Communicated by David Nachmansohn, February 2, 1968 The work of Weiss and his associates,1-3 and more recently of a number of other investigators,4- has established the occurrence of a flux of materials from the soma of neurons toward the peripheral regions of the axon. It has been postulated that this mechanism would account for the origin of most of the axonal protein, although the time required to cover the distance which separates some axonal tips from their cell bodies would impose severe delays.4 On the other hand, a number of observations7-9 have indicated the occurrence of local mechanisms of synthesis in peripheral axons, as suggested by the kinetics of appearance of individual proteins after axonal transection. In this paper we report the incorporation of radioactive amino acids into the protein fraction of the axoplasm and of the axonal envelope obtained from giant axons of the squid. These axons are isolated essentially free from small fibers and connective tissue, and pure samples of axoplasm may be obtained by extru- sion of the axon. Incorporation of amino acids into axonal protein has recently been reported using systems from mammals'0 and fish."I Materials and Methods.-Giant axons of Loligo pealii were dissected and freed from small fibers: they were tied at both ends. Incubations were carried out at 18-20° in sea water previously filtered through Millipore which contained 5 mM Tris pH 7.8 and 10 Muc/ml of a mixture of 15 C'4-labeled amino acids (New England Nuclear Co., Boston, Mass.). -

Neuronal Growth and Death: Minireview Order and Disorder in the Axoplasm
Cell, Vol. 84, 663±666, March 8, 1996, Copyright 1996 by Cell Press Neuronal Growth and Death: Minireview Order and Disorder in the Axoplasm Don W. Cleveland no neurofilaments in axons as a consequence of prema- Ludwig Institute for Cancer Research ture translation termination in NF-L (Ohara et al., 1993). and Departments of Medicine and Neuroscience This results in no detectable NF-L protein, total absence University of California, San Diego of neurofilaments, and resultant axons that almost com- 9500 Gilman Drive pletely fail to grow radially. La Jolla, California 92093 A central limit to neurofilment-dependent radial growth is the velocity and quantity of components deliv- ered to axons by axonal transport. Transport is a neces- Neurons, whose long thin axonal processes represent sity for neurons, as protein synthesis is restricted to the conduits for electrical signaling, are the most asym- cell bodies and dendrites. Membrane-bound particles metric cells in nature. Asymmetry arises in two steps, in axons are trafficked rapidly in both directions using each mediated by different cytoskeletal elements. Initial ATP-dependent microtubule motors. The remaining neurite elongation utilizes actin/myosin for growth cone components, including all known cytoskeletal proteins, locomotion and microtubules as tracks along which pro- teins and membranes are delivered from the cell body toward the developing axon terminus. After a stable synapse has formed, a second phase, termed radial growth, is initiated during which neurofilaments, the in- termediate filaments of most large neurons, accumulate to become the most abundant cytoskeletal elements (Figure 1B) and the axonal diameters increase by up to an order of magnitude (leading to up to a 100-fold in- crease in volume!). -

Regulation of Myelin Structure and Conduction Velocity by Perinodal Astrocytes
Correction NEUROSCIENCE Correction for “Regulation of myelin structure and conduc- tion velocity by perinodal astrocytes,” by Dipankar J. Dutta, Dong Ho Woo, Philip R. Lee, Sinisa Pajevic, Olena Bukalo, William C. Huffman, Hiroaki Wake, Peter J. Basser, Shahriar SheikhBahaei, Vanja Lazarevic, Jeffrey C. Smith, and R. Douglas Fields, which was first published October 29, 2018; 10.1073/ pnas.1811013115 (Proc. Natl. Acad. Sci. U.S.A. 115,11832–11837). The authors note that the following statement should be added to the Acknowledgments: “We acknowledge Dr. Hae Ung Lee for preliminary experiments that informed the ultimate experimental approach.” Published under the PNAS license. Published online June 10, 2019. www.pnas.org/cgi/doi/10.1073/pnas.1908361116 12574 | PNAS | June 18, 2019 | vol. 116 | no. 25 www.pnas.org Downloaded by guest on October 2, 2021 Regulation of myelin structure and conduction velocity by perinodal astrocytes Dipankar J. Duttaa,b, Dong Ho Wooa, Philip R. Leea, Sinisa Pajevicc, Olena Bukaloa, William C. Huffmana, Hiroaki Wakea, Peter J. Basserd, Shahriar SheikhBahaeie, Vanja Lazarevicf, Jeffrey C. Smithe, and R. Douglas Fieldsa,1 aSection on Nervous System Development and Plasticity, The Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892; bThe Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Bethesda, MD 20817; cMathematical and Statistical Computing Laboratory, Office of Intramural Research, Center for Information -

Autonomic Nervous System
Editing file Lecture 4: AUTONOMIC NERVOUS SYSTEM • Red : important • Pink : in girls slides only • Blue : in male slides only • Green : notes, Extra Objectives At the end of the lecture, students should be able to: ❖ Define the autonomic nervous system. ❖ Describe the structure of autonomic nervous system ❖ Trace the preganglionic & postganglionic neurons in both sympathetic & parasympathetic nervous system. ❖ Enumerate in brief the main effects of sympathetic & parasympathetic system Autonomic Nervous System The autonomic nervous system is concerned with the Autonomic nervous system: Nerve cells innervation and control of Involuntary structures such as located in both central & visceral organs, smooth muscles, cardiac muscles and glands. peripheral nervous system Skeletal muscles are controlled by somatic motor Difference between somatic and visceral motor: ● Somatic motor ● Function: Maintaining the homeostasis Fibers from Anterior horn cell —-> to target of the internal environment along with ● Visceral motor Regulation: (Controlled) the endocrine system. 1-Brain: from nuclei by the Hypothalamus 2- spinal cord: lateral horn cell Note: Hypothalamus controls ﺗﻌدي ﻋﻠﻰ . Ganglion ﻗﺑل ﺗوﺻل ﻟﻠـ Location: Central nervous system and Target ● both of Autonomic system + peripheral nervous system Endocrine system. Autonomic Nervous System Unlike the somatic nervous system, the Efferent pathway of the autonomic nervous system is made up of Preganglionic Neuron two neurons called as: Preganglionic Postganglionic The cell bodies are The cell bodies are Postganglionic Neuron located in the brain located in the and spinal cord autonomic ganglia (inside CNS ). (outside CNS). Preganglionic axons synapse with the postganglionic neurons Note: before the fibers reach the target, it should first pass by the autonomic ganglion and synapse ( interconnection).