Modulatory Roles of ATP and Adenosine in Cholinergic Neuromuscular Transmission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

P2Y6 Receptors Regulate CXCL10 Expression and Secretion in Mouse Intestinal Epithelial Cells
fphar-09-00149 February 26, 2018 Time: 17:57 # 1 ORIGINAL RESEARCH published: 28 February 2018 doi: 10.3389/fphar.2018.00149 P2Y6 Receptors Regulate CXCL10 Expression and Secretion in Mouse Intestinal Epithelial Cells Mabrouka Salem1,2, Alain Tremblay2, Julie Pelletier2, Bernard Robaye3 and Jean Sévigny1,2* 1 Département de Microbiologie-Infectiologie et d’Immunologie, Faculté de Médecine, Université Laval, Québec City, QC, Canada, 2 Centre de Recherche du CHU de Québec – Université Laval, Québec City, QC, Canada, 3 Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire, Université Libre de Bruxelles, Gosselies, Belgium In this study, we investigated the role of extracellular nucleotides in chemokine (KC, MIP- 2, MCP-1, and CXCL10) expression and secretion by murine primary intestinal epithelial cells (IECs) with a focus on P2Y6 receptors. qRT-PCR experiments showed that P2Y6 was the dominant nucleotide receptor expressed in mouse IEC. In addition, the P2Y6 Edited by: ligand UDP induced expression and secretion of CXCL10. For the other studies, we Kenneth A. Jacobson, −=− National Institutes of Health (NIH), took advantage of mice deficient in P2Y6 (P2ry6 ). Similar expression levels of P2Y1, −=− United States P2Y2, P2X2, P2X4, and A2A were detected in P2ry6 and WT IEC. Agonists of Reviewed by: TLR3 (poly(I:C)), TLR4 (LPS), P2Y1, and P2Y2 increased the expression and secretion Fernando Ochoa-Cortes, of CXCL10 more prominently in P2ry6−=− IEC than in WT IEC. CXCL10 expression Universidad Autónoma de San Luis −=− Potosí, Mexico and secretion induced by poly(I:C) in both P2ry6 and WT IEC were inhibited by Markus Neurath, general P2 antagonists (suramin and Reactive-Blue-2), by apyrase, and by specific Universitätsklinikum Erlangen, Germany antagonists of P2Y1, P2Y2, P2Y6 (only in WT), and P2X4. -

Functional Selectivity and Partial Efficacy at the Monoamine Transporters: a Unified Model of Allosteric Modulation and Amphetamine-Induced Substrate Release
1521-0111/95/3/303–312$35.00 https://doi.org/10.1124/mol.118.114793 MOLECULAR PHARMACOLOGY Mol Pharmacol 95:303–312, March 2019 Copyright ª 2019 by The Author(s) This is an open access article distributed under the CC BY-NC Attribution 4.0 International license. MINIREVIEW Functional Selectivity and Partial Efficacy at the Monoamine Transporters: A Unified Model of Allosteric Modulation and Amphetamine-Induced Substrate Release Peter S. Hasenhuetl,1,2 Shreyas Bhat,2 Michael Freissmuth, and Walter Sandtner Downloaded from Institute of Pharmacology, Gaston H. Glock Research Laboratories for Exploratory Drug Development, Center of Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria Received October 2, 2018; accepted December 13, 2018 ABSTRACT molpharm.aspetjournals.org All clinically approved drugs targeting the plasmalemmal trans- allosteric inhibition and stimulation of MATs, which can be porters for dopamine, norepinephrine, and serotonin act either functionally selective for either substrate uptake or amphetamine- as competitive uptake inhibitors or as amphetamine-like releas- induced release. These concepts are integrated into a parsimonious ers. Monoamine transporter (MAT) ligands that allosterically kinetic framework, which relies exclusively on physiologic transport affect MAT-mediated substrate uptake, release, or both were modes (without any deviation from an alternating access mecha- recently discovered. Their modes of action have not yet been nism). The model posits cooperative substrate and Na1 binding and explained in a unified framework. Here, we go beyond compet- functional selectivity by conformational selection (i.e., preference of itive inhibitors and classic amphetamines and introduce con- the allosteric modulators for the substrate-loaded or substrate-free cepts for partial efficacy at and allosteric modulation of MATs. -

Mechanisms of Acetylcholine Receptor Loss in Myasthenia Gravis
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.43.7.601 on 1 July 1980. Downloaded from Journal of Neurology, Neurosurgery, and Psychiatry, 1980, 43, 601-610 Mechanisms of acetylcholine receptor loss in myasthenia gravis DANIEL B DRACHMAN, ROBERT N ADAMS, ELIS F STANLEY, AND ALAN PESTRONK From the Department of Neurology, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA SUMMARY The fundamental abnormality affecting the neuromuscular junctions of myasthenic patients is a reduction of available AChRs, due to an autoimmune attack directed against the receptors. Antibodies to AChR are present in most patients, and there is evidence that they have a predominant pathogenic role in the disease, aided by complement. The mechanism of antibody action involves acceleration of the rate of degradation of AChRs, attributable to cross-linking of the receptors. In addition, antibodies may block AChRs, and may participate in producing destructive changes, perhaps in conjunction with complement. The possibility that cell-mediated mechanisms may play a role in the autoimmune responses of some myasthenic patients remains to be explored. Although the target of the autoimmune attack in myasthenic patients is probably always the acetyl- Protected by copyright. choline receptors, it is not yet clear which of these immune mechanisms are most important. It is likely that the relative role of each mechanism varies from patient to patient. One of the goals of future research will be to identify the relative importance of each -

Ageing, Sex and Cardioprotection Marisol Ruiz-Meana1,2, Kerstin Boengler3, David Garcia-Dorado1,2, Derek J
Kaambre Tuuli (Orcid ID: 0000-0001-5755-4694) Kararigas Georgios (Orcid ID: 0000-0002-8187-0176) Ageing, sex and cardioprotection Marisol Ruiz-Meana1,2, Kerstin Boengler3, David Garcia-Dorado1,2, Derek J. Hausenloy4,5,6,7,8,9, Tuuli Kaambre10, Georgios Kararigas11,12, Cinzia Perrino13, Rainer Schulz3, Kirsti Ytrehus14. 1 Hospital Universitari Vall d’Hebron, Department of Cardiology. Vall d’Hebron Institut de Recerca (VHIR).Universitat Autonoma de Barcelona, Spain. 2 Centro de Investigación Biomédica en Red-CV, CIBER-CV, Spain. 3Institute of Physiology, Justus-Liebig University Giessen. Giessen 35392, Aulweg 129, Germany. 4Cardiovascular & Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore. 5National Heart Research Institute Singapore, National Heart Centre, Singapore. 6Yong Loo Lin School of Medicine, National University Singapore, Singapore. 7The Hatter Cardiovascular Institute, University College London, London, UK. 8The National Institute of Health Research University College London Hospitals Biomedical Research Centre, Research & Development, London, UK. 9Tecnologico de Monterrey, Centro de Biotecnologia-FEMSA, Nuevo Leon, Mexico. 10Laboratory of Chemical Biology, National Institute of Chemical Physics and Biophysics, Akadeemia tee 23, 12618 Tallinn, Estonia. 11Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany. 12DZHK (German Centre for Cardiovascular Research), partner site Berlin, -

Protease Effects on the Structure of Acetylcholine Receptor Membranes from Torpedo Californica
PROTEASE EFFECTS ON THE STRUCTURE OF ACETYLCHOLINE RECEPTOR MEMBRANES FROM TORPEDO CALIFORNICA MICHAEL W. KLYMKOWSKY, JOHN E . HEUSER, and ROBERT M. STROUD From the Department of Biochemistry & Biophysics, University of California at San Francisco, San Francisco, California 94143 . Dr . Klymkowsky's present address is MRC Neuroimmunology Project, Department of Zoology, University College London, London WC IE, 6BT, England ABSTRACT Protease digestion of acetylcholine receptor-rich membranes derived from Torpedo californica electroplaques by homogenization and isopycnic centrifugation results in degradation of all receptor subunits without any significant effect on the appearance in electron micrographs, the toxin binding ability, or the sedimentation value of the receptor molecule . Such treatment does produce dramatic changes in the morphology of the normally 0.5- to 2-lm-diameter spherical vesicles when observed by either negative-stain or freeze-fracture electron microscopy . Removal of peripheral, apparently nonreceptor polypeptides by alkali stripping (Neubig et al ., 1979, Proc. Natl. Acad. Sci. U. S. A. 76:690-694) results in increased sensitivity of the acetylcholine receptor membranes to the protease trypsin as indicated by SDS gel electrophoretic patterns and by the extent of morphologic change observed in vesicle structure . Trypsin digestion of alkali-stripped receptor membranes results in a limit degradation pattern of all four receptor subunits, whereupon all the vesicles undergo the morphological transformation to minivesicles -

Interplay Between Gating and Block of Ligand-Gated Ion Channels
brain sciences Review Interplay between Gating and Block of Ligand-Gated Ion Channels Matthew B. Phillips 1,2, Aparna Nigam 1 and Jon W. Johnson 1,2,* 1 Department of Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA; [email protected] (M.B.P.); [email protected] (A.N.) 2 Center for Neuroscience, University of Pittsburgh, Pittsburgh, PA 15260, USA * Correspondence: [email protected]; Tel.: +1-(412)-624-4295 Received: 27 October 2020; Accepted: 26 November 2020; Published: 1 December 2020 Abstract: Drugs that inhibit ion channel function by binding in the channel and preventing current flow, known as channel blockers, can be used as powerful tools for analysis of channel properties. Channel blockers are used to probe both the sophisticated structure and basic biophysical properties of ion channels. Gating, the mechanism that controls the opening and closing of ion channels, can be profoundly influenced by channel blocking drugs. Channel block and gating are reciprocally connected; gating controls access of channel blockers to their binding sites, and channel-blocking drugs can have profound and diverse effects on the rates of gating transitions and on the stability of channel open and closed states. This review synthesizes knowledge of the inherent intertwining of block and gating of excitatory ligand-gated ion channels, with a focus on the utility of channel blockers as analytic probes of ionotropic glutamate receptor channel function. Keywords: ligand-gated ion channel; channel block; channel gating; nicotinic acetylcholine receptor; ionotropic glutamate receptor; AMPA receptor; kainate receptor; NMDA receptor 1. Introduction Neuronal information processing depends on the distribution and properties of the ion channels found in neuronal membranes. -

P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases
International Journal of Molecular Sciences Review P2Y Purinergic Receptors, Endothelial Dysfunction, and Cardiovascular Diseases Derek Strassheim 1, Alexander Verin 2, Robert Batori 2 , Hala Nijmeh 3, Nana Burns 1, Anita Kovacs-Kasa 2, Nagavedi S. Umapathy 4, Janavi Kotamarthi 5, Yash S. Gokhale 5, Vijaya Karoor 1, Kurt R. Stenmark 1,3 and Evgenia Gerasimovskaya 1,3,* 1 The Department of Medicine Cardiovascular and Pulmonary Research Laboratory, University of Colorado Denver, Aurora, CO 80045, USA; [email protected] (D.S.); [email protected] (N.B.); [email protected] (V.K.); [email protected] (K.R.S.) 2 Vascular Biology Center, Augusta University, Augusta, GA 30912, USA; [email protected] (A.V.); [email protected] (R.B.); [email protected] (A.K.-K.) 3 The Department of Pediatrics, Division of Critical Care Medicine, University of Colorado Denver, Aurora, CO 80045, USA; [email protected] 4 Center for Blood Disorders, Augusta University, Augusta, GA 30912, USA; [email protected] 5 The Department of BioMedical Engineering, University of Wisconsin, Madison, WI 53706, USA; [email protected] (J.K.); [email protected] (Y.S.G.) * Correspondence: [email protected]; Tel.: +1-303-724-5614 Received: 25 August 2020; Accepted: 15 September 2020; Published: 18 September 2020 Abstract: Purinergic G-protein-coupled receptors are ancient and the most abundant group of G-protein-coupled receptors (GPCRs). The wide distribution of purinergic receptors in the cardiovascular system, together with the expression of multiple receptor subtypes in endothelial cells (ECs) and other vascular cells demonstrates the physiological importance of the purinergic signaling system in the regulation of the cardiovascular system. -

NIH Public Access Author Manuscript Neuron Glia Biol
NIH Public Access Author Manuscript Neuron Glia Biol. Author manuscript; available in PMC 2006 May 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Neuron Glia Biol. 2006 May ; 2(2): 125±138. Purinergic receptors activating rapid intracellular Ca2+ increases in microglia Alan R. Light1, Ying Wu2, Ronald W. Hughen1, and Peter B. Guthrie3 1 Department of Anesthesiology, University of Utah, Salt Lake City, UT, USA 2 Oral Biology Program, School of Dentistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27510, USA 3 Scientific Review Administrator, Center for Scientific Review, National Institutes of Health, 6701 Rockledge Drive, Room 4142 Msc 7850, Bethesda, MD 20892-7850, USA Abstract We provide both molecular and pharmacological evidence that the metabotropic, purinergic, P2Y6, P2Y12 and P2Y13 receptors and the ionotropic P2X4 receptor contribute strongly to the rapid calcium response caused by ATP and its analogues in mouse microglia. Real-time PCR demonstrates that the most prevalent P2 receptor in microglia is P2Y6 followed, in order, by P2X4, P2Y12, and P2X7 = P2Y13. Only very small quantities of mRNA for P2Y1, P2Y2, P2Y4, P2Y14, P2X3 and P2X5 were found. Dose-response curves of the rapid calcium response gave a potency order of: 2MeSADP>ADP=UDP=IDP=UTP>ATP>BzATP, whereas A2P4 had little effect. Pertussis toxin partially blocked responses to 2MeSADP, ADP and UDP. The P2X4 antagonist suramin, but not PPADS, significantly blocked responses to ATP. These data indicate that P2Y6, P2Y12, P2Y13 and P2X receptors mediate much of the rapid calcium responses and shape changes in microglia to low concentrations of ATP, presumably at least partly because ATP is rapidly hydrolyzed to ADP. -

Cellular Trafficking of Nicotinic Acetylcholine Receptors
npg Acta Pharmacol Sin 2009 Jun; 30 (6): 656–662 Review Cellular trafficking of nicotinic acetylcholine receptors Paul A ST JOHN* Department of Cell Biology and Anatomy, University of Arizona College of Medicine, Tucson, AZ 85724, USA Nicotinic acetylcholine receptors (nAChRs) play critical roles throughout the body. Precise regulation of the cellular loca- tion and availability of nAChRs on neurons and target cells is critical to their proper function. Dynamic, post-translational regulation of nAChRs, particularly control of their movements among the different compartments of cells, is an important aspect of that regulation. A combination of new information and new techniques has the study of nAChR trafficking poised for new breakthroughs. Keywords: membrane traffic; protein traffic; biosynthesis; endocytosis; endoplasmic reticulum-associated degradation Acta Pharmacologica Sinica (2009) 30: 656–662; doi: 10.1038/aps.2009.76 Introduction ways, but two particular perturbations have been especially well studied and exert their effects at least in part by altering Nicotinic acetylcholine receptors (nAChRs) mediate the trafficking of nAChRs: 1) denervation changes the total synaptic transmission in the CNS, in autonomic ganglia, and number, the distribution, and the turnover rate of nAChRs in at neuromuscular junctions and other peripheral synapses. skeletal muscle; 2) prolonged exposure to nicotine increases The functional properties of these synapses differ, but in each the total number of nAChRs in neurons. Several of the stud- case, properly functional signaling requires cellular control ies reviewed here addressed the mechanisms by which these of the number, type, and location of nAChRs. Trafficking treatments alter nAChR trafficking. Other authors in this of nAChRs – the movement of nAChRs between compart- special issue will address other aspects of the effects of nico- ments of a cell, including the cell's biosynthetic and degrada- tine on nAChRs. -
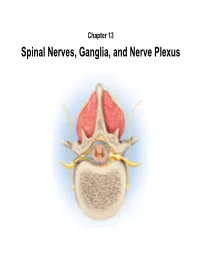
Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves
Chapter 13 Spinal Nerves, Ganglia, and Nerve Plexus Spinal Nerves Posterior Spinous process of vertebra Posterior root Deep muscles of back Posterior ramus Spinal cord Transverse process of vertebra Posterior root ganglion Spinal nerve Anterior ramus Meningeal branch Communicating rami Anterior root Vertebral body Sympathetic ganglion Anterior General Anatomy of Nerves and Ganglia • Spinal cord communicates with the rest of the body by way of spinal nerves • nerve = a cordlike organ composed of numerous nerve fibers (axons) bound together by connective tissue – mixed nerves contain both afferent (sensory) and efferent (motor) fibers – composed of thousands of fibers carrying currents in opposite directions Anatomy of a Nerve Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Epineurium Perineurium Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Endoneurium Nerve Rootlets fiber Posterior root Fascicle Posterior root ganglion Anterior Blood root vessels Spinal nerve (b) Copyright by R.G. Kessel and R.H. Kardon, Tissues and Organs: A Text-Atlas of Scanning Electron Microscopy, 1979, W.H. Freeman, All rights reserved Blood vessels Fascicle Epineurium Perineurium Unmyelinated nerve fibers Myelinated nerve fibers (a) Endoneurium Myelin General Anatomy of Nerves and Ganglia • nerves of peripheral nervous system are ensheathed in Schwann cells – forms neurilemma and often a myelin sheath around the axon – external to neurilemma, each fiber is surrounded by -

Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor
Genes 2013, 4, 171-197; doi:10.3390/genes4020171 OPEN ACCESS genes ISSN 2073-4425 www.mdpi.com/journal/genes Review Non-Neuronal Functions of the M2 Muscarinic Acetylcholine Receptor Wymke Ockenga, Sina Kühne, Simone Bocksberger, Antje Banning and Ritva Tikkanen * Institute of Biochemistry, Medical Faculty, University of Giessen, Friedrichstrasse 24, 35392 Giessen, Germany; E-Mails: [email protected] (W.O.); [email protected] (S.K.); [email protected] (S.B.); [email protected] (A.B.) * Author to whom correspondence should be addressed; E-Mail: [email protected]; Tel.: +49-641-9947-420; Fax: +49-641-9947-429. Received: 31 January 2013; in revised form: 10 March 2013 / Accepted: 25 March 2013 / Published: 2 April 2013 Abstract: Acetylcholine is an important neurotransmitter whose effects are mediated by two classes of receptors. The nicotinic acetylcholine receptors are ion channels, whereas the muscarinic receptors belong to the large family of G protein coupled seven transmembrane helix receptors. Beyond its function in neuronal systems, it has become evident that acetylcholine also plays an important role in non-neuronal cells such as epithelial and immune cells. Furthermore, many cell types in the periphery are capable of synthesizing acetylcholine and express at least some of the receptors. In this review, we summarize the non-neuronal functions of the muscarinic acetylcholine receptors, especially those of the M2 muscarinic receptor in epithelial cells. We will review the mechanisms of signaling by the M2 receptor but also the cellular trafficking and ARF6 mediated endocytosis of this receptor, which play an important role in the regulation of signaling events. -

Full-Text PDF (Final Published Version)
Alexander, S. P. H., Cidlowski, J. A., Kelly, E., Marrion, N. V., Peters, J. A., Faccenda, E., Harding, S. D., Pawson, A. J., Sharman, J. L., Southan, C., Davies, J. A., & CGTP Collaborators (2017). THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology, 174, S208-S224. https://doi.org/10.1111/bph.13880 Publisher's PDF, also known as Version of record License (if available): CC BY Link to published version (if available): 10.1111/bph.13880 Link to publication record in Explore Bristol Research PDF-document This is the final published version of the article (version of record). It first appeared online via Wiley at https://doi.org/10.1111/bph.13880 . Please refer to any applicable terms of use of the publisher. University of Bristol - Explore Bristol Research General rights This document is made available in accordance with publisher policies. Please cite only the published version using the reference above. Full terms of use are available: http://www.bristol.ac.uk/red/research-policy/pure/user-guides/ebr-terms/ S.P.H. Alexander et al. The Concise Guide to PHARMACOLOGY 2017/18: Nuclear hormone receptors. British Journal of Pharmacology (2017) 174, S208–S224 THE CONCISE GUIDE TO PHARMACOLOGY 2017/18: Nuclear hormone receptors Stephen PH Alexander1, John A Cidlowski2, Eamonn Kelly3, Neil V Marrion3, John A Peters4, Elena Faccenda5, Simon D Harding5,AdamJPawson5, Joanna L Sharman5, Christopher Southan5, Jamie A Davies5 and CGTP Collaborators 1School of Life Sciences, University of Nottingham Medical