With Laparotomy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gastrostomy Feeding Tubes
Gastrostomy feeding tubes With Dr Anastasia Volovets, Gastroenterologist and Hepatologist, Royal Prince Alfred Hospital, Sydney, Australia Introduction In patients with prolonged inadequate or absent oral intake gastrostomy tubes can be used to provide a route for enteral feeding, hydration, and medication administration. Case 1 - You are a junior doctor on the wards and you’re called to see a 65 year old male, who is day 5 post- stroke with an impaired swallow he is unable to tolerate oral feed and his family is worried he will starve to death. 1. Management of this patient IV fluids do not provide the caloric support or nutrients needed by patients, after 48 hours of impaired oral feeding, enteral feeding should be considered. • Short term this would be a nasogastric tube • Longer term (greater than 6 weeks) a gastrostomy or jejunostomy should be considered 2. Indications for enteral feeding • Neurological disorders causing impaired swallowing and aspiration of food o Stroke (most common) o Traumatic brain injury o Parkinson’s disease • Structural problems o Malignancy obstructing the gastrointestinal tract, this can include upper GI, head, nose or throat. Gastrostomy insertion can be done prophylactically prior to treatment that will impair the functioning or path of the tract such as surgery or radiotherapy 3. Contraindications to gastric feeding tubes • Absolute o High bleeding risk - uncorrected coagulopathy, thrombocytopenia o Chronic liver disease - varies and ascites o Peritonitis or abdominal perforation o Cellulitis at selected -

Pancreaticogastrostomy
eCommons@AKU Section of General Surgery Department of Surgery October 2017 Pancreaticogastrostomy - an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy - experience from a tertiary care center of Pakistan Tabish Chawla Aga Khan University, [email protected] Hassaan Bari Aga Khan University Shahrukh Effendi Follow this and additional works at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_gen Part of the Surgery Commons Recommended Citation Chawla, T., Bari, H., Effendi, S. (2017). Pancreaticogastrostomy - an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy - experience from a tertiary care center of Pakistan. Journal of Pakistan Medical Association, 67(10), 1621-1624. Available at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_gen/76 1621 CASE SERIES Pancreaticogastrostomy — an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy — experience from a tertiary care center of Pakistan Tabish Chawla, Hassaan Bari, Shahrukh Effendi Abstract as part of PD. Therefore it was associated with high Whipple's pancreaticoduodenectomy has been refined morbidity and mortality resulting from high rates of over the years to be a safe operation though the leakage from pancreatic stump. morbidity rate still remains high (30-50%). Pancreatic Pancreatcogastrostomy is a repopularized technique fistula is the most important cause of mortality which has been described previously in literature. 3 This following pancreaticoduodenectomy. To prevent it, study was done to review the experience of PG being surgeons have used two anastomotic techniques: done as an alternate to PJ after PD. pancreaticojejunostomy and pancreaticogastrostomy. Recent studies found that pancreaticogastrostomy is Material and Methods associated with fewer overall complications than It is a case series collected at the Department of Surgery of pancreaticojejunostomy. -

High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider
NUTRITIONINFLAMMATORY ISSUES BOWEL IN GASTROENTEROLOGY, DISEASE: A PRACTICAL SERIES APPROACH, #105 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider Iris Vance Neeral Shah Percutaneous endoscopy gastrostomy (PEG) tubes are a valuable tool for providing long- term enteral nutrition or gastric decompression; certain circumstances that complicate PEG placement warrant novel approaches and merit review and discussion. Ascites and portal hypertension with varices have been associated with poorer outcomes. Bleeding is one of the most common serious complications affecting approximately 2.5% of all procedures. This article will review what evidence exists in these high risk scenarios and attempt to provide more clarity when considering these challenging clinical circumstances. INTRODUCTION ince the first Percutaneous Endoscopic has been found by multiple authors to portend a poor Gastrostomy tube was placed in 1979 (1), they prognosis in PEG placement (3,4, 5,6,7,8). This review Shave become an invaluable tool for providing will endeavor to provide more clarity when considering long-term enteral nutrition (EN) and are commonly used these challenging clinical circumstances. in patients with dysphagia following stroke, disabling motor neuron diseases such as multiple sclerosis and Ascites & Gastric Varices amyotrophic lateral sclerosis, and in those with head The presence of ascites is frequently viewed as a and neck cancer.They are also used for patients with relative, if not absolute, contraindication to PEG prolonged mechanical intubation, as well as gastric placement. Ascites adds technical difficulties and the decompression in those with severe gastroparesis, risk for potential complications (see Table 1). -
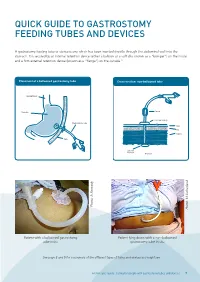
Quick Guide to Gastrostomy Feeding Tubes and Devices
QUICK GUIDE TO GASTROSTOMY FEEDING TUBES AND DEVICES A gastrostomy feeding tube or device is one which has been inserted directly through the abdominal wall into the stomach. It is secured by an internal retention device (either a balloon or a soft disc known as a “bumper”) on the inside and a firm external retention device (known as a “flange”) on the outside.11 Placement of a ballooned gastrostomy tube Cross-section: non-ballooned tube Oesophagus Stomach Clamp External Flange Gastrostomy tube Skin Fat Muscle Skin Internal Bumper Stomach Photo: APhoto: Kennedy Photo: MPhoto: Sutherland Patient with a ballooned gastrostomy Patient lying down with a non-ballooned tube insitu gastrostomy tube in situ See page 8 and 9 for a summary of the different types of tubes and devices you might see. A Clinician’s Guide: Caring for people with gastrostomy tubes and devices 7 Common features of gastrostomy feeding tubes and devices include, but are not limited to: Refer to manufacturer’s guidelines for advice on brand specific tube and device features Ballooned Gastrostomy Tube Ballooned Gastrostomy Tube With side port Without side port Feeding Port Feeding Port (Enteral Dispenser (Enteral Dispenser and Feed Bag and Feed Bag connect here) connect here) ml/cc Balloon Port Balloon Port ml/cc Side Port (X ml/cc) (X ml/cc) French (size) [For example:16/18/20] French (size) [For example:16/18/20] FR FR cm markings cm markings External External Flange Flange Balloon Balloon Non-ballooned Gastrostomy Tube Non-ballooned Gastrostomy Tube with collapsible internal -

The Place of Laparoscopic Gastrostomy in the Surgical Armamentarium
6 The Place of Laparoscopic Gastrostomy in the Surgical Armamentarium Philip Ng Cheng Hin Department Of Surgery, University Hospital Lewisham, London UK 1. Introduction Historically, gastrostomy has been performed for centuries and recently with the advent of Laparoscopic surgery, laparoscopic gastrostomy (ref 1) has been added to the options available to surgeons. Laparoscopic gastrostomy can be considered when other minimally invasive methods such as PEG (Percutaneous Endoscopic Gastrostomy) is not feasible or fails. PEG can be come impossible if the endoscope cannot be introduced, because of physical or functional obstruction. The alternative is to consider PRG (Percutaneous Radiologically Guided) insertion prior to considering open gastrostomy via laparotomy, (LG) Laparoscopic gastrostomy has carved itself an important niche in that respect. 2. Indications of gastrostomy a. Patients requiring Medium or long term feeding - Starvation - Swallowing problems, long term neurological conditions - Chronic problems in children e.g. mucoviscidosis, reflux - Impassable benign or malignant stricture b. Decompression of the stomach c. Gastric access d. Failure of PEG e. Failure of PRG 3. Contra indications a. Unfit patients who cannot lie flat. b. Abdominal access not possible due to previous operations or gross obesity, fixed flexion deformity. 4. Techniques a. Double puncture laparoscopic assisted gastrostomy (ref 2, 4) Once the abdomen prepped, entry into the peritoneal cavity is performed using any preferred technique through a periumbilical port and the pneumoperitoneum is www.intechopen.com 84 Gastrostomy established, the anterior wall of the stomach is identified with certainty, and a second port (10mm) is inserted at a convenient point on the anterior abdominal wall. This operative step is greatly assisted by changing the position of the operating table 20 degrees head up. -

Gastrostomy Allows Removal of Obstructive Pancreatic Duct Stones
Original article Antegrade pancreatoscopy via EUS-guided pancreatico- gastrostomy allows removal of obstructive pancreatic duct stones Authors Theodore W. James, Todd H. Baron Institution pancreaticolithiasis, including use of pancreatoscopy for in- Division of Gastroenterology and Hepatology, University of traductal electrohydraulic lithotripsy (IEHL). Pancreatosco- North Carolina, Chapel Hill, North Carolina, United States py is often limited by a small-caliber downstream pancreat- ic duct as well as an unstable pancreatoscope position submitted 8.3.2018 within the pancreatic head. Endoscopic ultrasound-guided accepted after revision 11.4.2018 pancreaticogastrostomy (EUS-PG) has been developed as a method to relieve ductal obstruction when retrograde ac- Bibliography cess fails. The current study describes pancreatoscopy via DOI https://doi.org/10.1055/a-0607-2484 | EUS-PG, a novel method for managing obstructing pancrea- Endoscopy International Open 2018; 06: E735–E738 ticolithiasis. © Georg Thieme Verlag KG Stuttgart · New York Patients and methods From September 2017 to January ISSN 2364-3722 2018, patients who underwent EUS-PG followed by ante- grade pancreatoscopy via PG were identified. Endoscopy re- Corresponding author ports, medical charts and relevant laboratory data were re- Todd Huntley Baron, MD, Division of Gastroenterology and viewed and recorded. Hepatology, University of North Carolina School of Results Five patients underwent EUS-PG and antegrade Medicine, 101 Manning Drive, Chapel Hill, NC 27599 pancreatoscopy via PG during the study period; clinical suc- Fax: +1-984-974-0744 cess rate was 100%. There were no significant adverse [email protected] events during the procedure or follow up period. Conclusions Pancreatoscopy via PG for IEHL is safe and ef- fective for treating obstructing pancreaticolithiasis in pa- ABSTRACT tients who have previously failed ERCP or in clinical scenar- Background and study aims Endoscopic retrograde cho- ios were ERCP is not possible. -

Laparoscopic Intracorporeal Pancreaticogastrostomy in Total Laparoscopic Pancreaticoduodenectomy—A Novel Anastomotic Technique
Indian Journal of Surgical Oncology (June 2019) 10(2):274–279 https://doi.org/10.1007/s13193-018-0829-4 ORIGINAL ARTICLE Laparoscopic Intracorporeal Pancreaticogastrostomy in Total Laparoscopic Pancreaticoduodenectomy—A Novel Anastomotic Technique Shailesh P. Puntambekar1 & Mehul J. Mehta1 & Manoj M. Manchekar1 & Mihir Chitale1 & Mangesh Panse1 & Advait Jathar1 & Rohan Umalkar1 Received: 8 March 2018 /Accepted: 13 November 2018 /Published online: 2 January 2019 # Indian Association of Surgical Oncology 2019 Abstract Novel pancreaticogastric anastomosis technique in laparoscopic pancreaticoduodenectomy which is simple, feasible to perform, provides secure fixation between stomach and pancreas. The aim of our article is to describe our technique of intracorporeal pancreaticogastrostomy as a promising approach for future widespread application. Keywords PD-pancreaticoduocenectomy . PJ-pancreaticojejunostomy . PG-pancreaticogastrostomy Introduction aim of our article is to describe our technique of intracorporeal pancreaticogastrostomy as a promising approach for future Laparoscopic pancreatic surgery has emerged as one of the widespread application. most advanced applications of minimal invasive surgery. Gagner and Pomp were the first to describe the laparoscopic pancreaticoduodenectomy in 1994. Prolonged operative time Methods and technical difficulty of pancreatic resection and reconstruc- tion procedures were the reasons for initial reluctance to ac- We have used our technique in five patients since May 2015 to cept the laparoscopic technique. Pancreatic anastomotic leak- March 2016. The inclusion criteria were medically fit, non- age carries an increased risk of intraabdominal haemorrhage obese patients with periampullary tumours and without any and high mortality rate. Many surgeons avoid intracorporeal previous abdominal surgery. Preoperatively, all patients were pancreatic reconstruction to increase the safety of anastomo- thoroughly evaluated for operability and resectability. -

Percutaneous Endoscopic Gastrostomy Versus Nasogastric Tube Feeding: Oropharyngeal Dysphagia Increases Risk for Pneumonia Requiring Hospital Admission
nutrients Article Percutaneous Endoscopic Gastrostomy versus Nasogastric Tube Feeding: Oropharyngeal Dysphagia Increases Risk for Pneumonia Requiring Hospital Admission Wei-Kuo Chang 1,*, Hsin-Hung Huang 1, Hsuan-Hwai Lin 1 and Chen-Liang Tsai 2 1 Division of Gastroenterology, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei 114, Taiwan; [email protected] (H.-H.H.); [email protected] (H.-H.L.) 2 Division of Pulmonary and Critical Care, Department of Internal Medicine, Tri-Service General Hospital, National Defense Medical Center, Taipei 114, Taiwan; [email protected] * Correspondence: [email protected]; Tel.: +886-2-23657137; Fax: +886-2-87927138 Received: 3 November 2019; Accepted: 4 December 2019; Published: 5 December 2019 Abstract: Background: Aspiration pneumonia is the most common cause of death in patients with percutaneous endoscopic gastrostomy (PEG) and nasogastric tube (NGT) feeding. This study aimed to compare PEG versus NGT feeding regarding the risk of pneumonia, according to the severity of pooling secretions in the pharyngolaryngeal region. Methods: Patients were stratified by endoscopic observation of the pooling secretions in the pharyngolaryngeal region: control group (<25% pooling secretions filling the pyriform sinus), pharyngeal group (25–100% pooling secretions filling the pyriform sinus), and laryngeal group (pooling secretions entering the laryngeal vestibule). Demographic data, swallowing level scale score, and pneumonia requiring hospital admission were recorded. Results: Patients with NGT (n = 97) had a significantly higher incidence of pneumonia (episodes/person-years) than those patients with PEG (n = 130) in the pharyngeal group (3.6 1.0 ± vs. 2.3 2.1, P < 0.001) and the laryngeal group (3.8 0.5 vs. -

The Role of Interventional Radiology in the Management of Pancreatic Pathologies
Published online: 2020-01-10 THIEME Review Article 99 The Role of Interventional Radiology in the Management of Pancreatic Pathologies Ghali Salahia1 Sook Cheng Chin2 Ian Zealley2 Richard D. White1 1Department of Radiology, University Hospital of Wales, Cardiff, Address for correspondence Ghali Salahia, MD, MBA, Department United Kingdom of Radiology, University Hospital of Wales, Heath Park Way, Cardiff, 2Department of Radiology, Ninewells Hospital, Dundee, United CF14 4XW, United Kingdom (e-mail: [email protected]). Kingdom J Gastrointestinal Abdominal Radiol ISGAR 2020;3:99–114 Abstract Pancreatic pathologies are varied and wide-ranging, and a multidisciplinary approach is essential for effective diagnosis and management. We describe image-guided percutaneous (nonendoscopic) interventions in the management of pancreatic dis- ease, with emphasis on inflammatory and neoplastic pancreatic pathologies and on the transplanted pancreas. Image-guided treatments for the complications of pan- Keywords creatitis include percutaneous interventions on simple and complex peripancreatic ► interventional collections, pseudocysts, and fistulas. Vascular interventions predominantly focus on radiology the treatment of pseudoaneurysms, hemorrhagic pseudocysts, and arteriovenous ► pancreatic cancer malformations. Emerging ablative techniques for pancreatic cancer are promising ► pancreatic transplant and include percutaneous radiofrequency ablation, microwave ablation, irreversible ► pancreatitis electroporation, and electrochemotherapy. Image-guided -

Ex Vivo Resection and Autotransplantation for Pancreatic Neoplasms
Ex Vivo Resection and Autotransplantation for Pancreatic Neoplasms Peter Liou, MD, Tomoaki Kato, MD, MBA* KEYWORDS Pancreas Pancreatic tumors Ex vivo resection Autotransplantation Mesenteric root involvement SMA involvement KEY POINTS Ex vivo resection and autotransplantation is a technique derived from multivisceral and intestinal transplantation whereby tumor-infiltrated organs are removed en bloc and pre- served in the cold, followed by tumor resection and reimplantation of the remaining viscera. Advantages of ex vivo resection include tumor removal in a bloodless field while mini- mizing the risk of ischemic injury to the involved organs. Access to the mesenteric root is greatly facilitated with ex vivo resection, and allows for safe reconstruction of major vasculature while preserving visceral integrity. Certain low-grade, non-adenocarcinomatous pancreatic neoplasms involving the mesen- teric vessels where aggressive surgical resection would be warranted, may benefit from ex vivo resection. Although ex vivo resections have been performed for pancreatic adenocarcinomas with major arterial involvement, the associated morbidity is significant and benefit remains unclear. INTRODUCTION Pancreatic neoplasms are a heterogeneous group of tumors arising from the pancreas with distinct and varied clinical profiles.1 Although pancreatic adenocarcinoma remains by far the most common and deadliest of these, there are several low- grade or benign neoplasms that may benefit from aggressive, curative resection.2,3 Due to the proximity of the pancreas to major abdominal vasculature, these tumors can sometimes infiltrate these vessels and preclude complete or safe resection by conventional surgical technique. Ex vivo resection and autotransplantation, whereby Financial Disclosures: The authors have nothing to disclose. Department of Surgery, Columbia University Medical Center, 622 West 168 Street PH14-105, New York, NY 10032, USA * Corresponding author. -

Post-Whipple: a Practical Approach to Nutrition Management
NINFLAMMATORYUTRITION ISSUES BOWEL IN GASTROENTEROLO DISEASE: A PRACTICALGY, SERIES APPROACH, #108 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor Post-Whipple: A Practical Approach to Nutrition Management Nora Decher Amy Berry The classic pancreatoduodenectomy (PD), also known as the Kausch-Whipple, and the pylorus- preserving pancreatoduodenectomy (PPPD) are most commonly performed for treatment of pancreatic cancer and chronic pancreatitis. This highly complex surgery disrupts the coordination of tightly orchestrated digestive processes. This, in combination with a diseased gland, sets the patient up for nutritional complications such as altered motility (gastroparesis and dumping), pancreatic insufficiency, diabetes mellitus, nutritional deficiencies and bacterial overgrowth. Close monitoring and attention to these issues will help the clinician optimize nutritional status and help prevent potentially devastating complications. 63-year-old female, D.D., presented to the copper, zinc, selenium, and potentially thiamine University of Virginia Health System (UVAHS) (thiamine was repleted before serum levels were Awith weight loss and biliary obstruction. She was checked). A percutaneous endoscopic gastrostomy with diagnosed with a large pancreatic serous cystadenoma jejunal extension (PEG-J) was placed due to intolerance and underwent a pancreatoduodenectomy (PD) of gastric enteral nutrition (EN). After several more (standard Whipple procedure with partial gastrectomy) hospitalizations, prolonged rehabilitation in a nursing with posterior anastomosis and cholecystectomy. Seven home, 7 months of supplemental nocturnal EN, and months later she was admitted to UVAHS with nausea, treatment of pancreatic insufficiency with pancreatic vomiting, diarrhea and a severe weight loss of 47lbs enzymes (with her meals and EN), D.D. was able to (33% of her usual body weight). -

2020 GI Endoscopy Coding and Reimbursement Guide
2020 GI Endoscopy Coding and Reimbursement Guide Disclaimer: The information provided herein reflects Cook’s understanding of the procedure(s) and/or device(s) from sources that may include, but are not limited to, the CPT® coding system; Medicare payment systems; commercially available coding guides; professional societies; and research conducted by independent coding and reimbursement consultants. This information should not be construed as authoritative. The entity billing Medicare and/or third party payers is solely responsible for the accuracy of the codes assigned to the services and items in the medical record. Cook does not, and should not, have access to medical records, and therefore cannot recommend codes for specific cases. When you are making coding decisions, we encourage you to seek input from the AMA, relevant medical societies, CMS, your local Medicare Administrative Contractor and other health plans to which you submit claims. Cook does not promote the off-label use of its devices. The reimbursement rates provided are national Medicare averages published by CMS at the time this guide was created. Reimbursement rates may change due to addendum updates Medicare publishes throughout the year and may not be reflected on the guide. CPT © 2019 American Medical Association. All rights reserved. CPT is a registered trademark of the American Medical Association. If you have any questions, please contact our reimbursement team at 800.468.1379 or by e-mail at [email protected]. 2020 GI Endoscopy Guide Medicare Reimbursement