A Clinician's Guide: Caring for People with Gastrostomy Tubes and Devices
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Laparoscopic Gastropexy As a Preventative Measure for Gastric Dilation Volvulus in Canines
Laparoscopic Gastropexy as a Preventative Measure for Gastric Dilation Volvulus in Canines By: Erin O’Brien Advisors: Dr. Kimberly Boswell Board Certified Surgeon Southwest Michigan Animal Emergency Hospital Kalamazoo, MI Dr. Diane R. Kiino Ph.D. Kalamazoo College Health Science A paper submitted in partial fulfillment of the requirements for the degree of Bachelor of Arts at Kalamazoo College. 2010 ii ACKNOWLEDGEMENTS Over the summer I was able to intern at the Southwest Michigan Animal Emergency Hospital in Kalamazoo, MI. It was there that I was exposed to the emergency setting in veterinary medicine but also had the chance to observe surgeries done by Board Certified Surgeon, Dr. Kimberly Boswell. I would like to thank the entire staff at SWMAEH for teaching me a tremendous amount about veterinary medicine and allowing me to get as much hands on experience as possible. It was such a privilege to complete my internship at a hospital where I was able to learn so much about veterinary medicine in only ten weeks. I would also like to thank Dr. Boswell in particular, it was a gastropexy surgery I saw her perform during my internship that inspired the topic of this paper. Additionally I would like to acknowledge my advisor Dr. Diane Kiino for providing the direction I needed in choosing my paper topic. iii ABSTRACT Gastric Dilation Volvulus (GDV) is a fatal condition in canines especially those that are large or giant breeds. GDV results from the stomach distending and twisting on itself which when left untreated causes shock and ultimately death. The only method of prevention for GDV is a gastropexy, a surgical procedure that sutures the stomach to the abdominal wall to prevent volvulus or twisting. -

Gastrostomy Feeding Tubes
Gastrostomy feeding tubes With Dr Anastasia Volovets, Gastroenterologist and Hepatologist, Royal Prince Alfred Hospital, Sydney, Australia Introduction In patients with prolonged inadequate or absent oral intake gastrostomy tubes can be used to provide a route for enteral feeding, hydration, and medication administration. Case 1 - You are a junior doctor on the wards and you’re called to see a 65 year old male, who is day 5 post- stroke with an impaired swallow he is unable to tolerate oral feed and his family is worried he will starve to death. 1. Management of this patient IV fluids do not provide the caloric support or nutrients needed by patients, after 48 hours of impaired oral feeding, enteral feeding should be considered. • Short term this would be a nasogastric tube • Longer term (greater than 6 weeks) a gastrostomy or jejunostomy should be considered 2. Indications for enteral feeding • Neurological disorders causing impaired swallowing and aspiration of food o Stroke (most common) o Traumatic brain injury o Parkinson’s disease • Structural problems o Malignancy obstructing the gastrointestinal tract, this can include upper GI, head, nose or throat. Gastrostomy insertion can be done prophylactically prior to treatment that will impair the functioning or path of the tract such as surgery or radiotherapy 3. Contraindications to gastric feeding tubes • Absolute o High bleeding risk - uncorrected coagulopathy, thrombocytopenia o Chronic liver disease - varies and ascites o Peritonitis or abdominal perforation o Cellulitis at selected -

Laparoscopic Fundoplication with Double Sided Posterior Gastropexy: a Different Surgical Technique
View metadata, citation and similar papers at core.ac.uk ORIGINAL RESEARCH brought to you by CORE provided by Elsevier - Publisher Connector International Journal of Surgery 10 (2012) 532e536 Contents lists available at SciVerse ScienceDirect International Journal of Surgery journal homepage: www.theijs.com Original research Laparoscopic fundoplication with double sided posterior gastropexy: A different surgical technique Fahri Yetis¸ira,*, A. Ebru Salman b,Dogukan Durak a, Mehmet Kiliç c a Ataturk Research and Training Hospital, General Surgery Department, Turkey b Ataturk Research and Training Hospital, Anesthesiology and Reanimation Department, Turkey c Yildirim Beyazit University, General Surgery Department, Turkey article info abstract Article history: Background: Laparoscopic Nissen Fundoplication has become the gold standard surgical procedure for Received 18 April 2012 management of gastroesophageal reflux disease. Nissen fundoplication provides an effective barrier Received in revised form against reflux. The aim of this study was to evaluate early postoperative outcomes of a different surgical 3 August 2012 technique, laparoscopic fundoplication with double sided posterior gastropexy. Accepted 6 August 2012 Methods: Data of 46 patients who underwent laparoscopic fundoplication with double sided posterior Available online 21 August 2012 gastropexy between February 2010 and December 2011 were collected. Surgically, after Nissen fundoplication was completed, 2e4 sutures were passed through the uppermost parts of the posterior Keywords: Gastropexy and anterior wall of the gastric wrap and then passed gently 1 cm above the celiac artery from the denser fi Nissen fundoplication bers of uppermost part of the arcuate ligament. Demographic data, preoperative and postoperative Gastroesophageal reflux assesments of sympthomatic and functional outcomes of patients were recorded. -

Modified Heller´S Esophageal Myotomy Associated with Dor's
Crimson Publishers Research Article Wings to the Research Modified Heller´s Esophageal Myotomy Associated with Dor’s Fundoplication A Surgical Alternative for the Treatment of Dolico Megaesophagus Fernando Athayde Veloso Madureira*, Francisco Alberto Vela Cabrera, Vernaza ISSN: 2637-7632 Monsalve M, Moreno Cando J, Charuri Furtado L and Isis Wanderley De Sena Schramm Department of General Surgery, Brazil Abstracts The most performed surgery for the treatment of achalasia is Heller´s esophageal myotomy associated or no with anti-reflux fundoplication. We propose in cases of advanced megaesophagus, specifically in the dolico megaesophagus, a technical variation. The aim of this study was to describe Heller´s myotomy modified by Madureira associated with Dor´s fundoplication as an alternative for the treatment of dolico megaesophagus,Materials and methods: assessing its effectiveness at through dysphagia scores and quality of life questionnaires. *Corresponding author: proposes the dissection ofTechnical the esophagus Note describing intrathoracic, the withsurgical circumferential procedure and release presenting of it, in the the results most of three patients with advanced dolico megaesophagus, operated from 2014 to 2017. The technique A. V. Madureira F, MsC, Phd. Americas Medical City Department of General extensive possible by trans hiatal route. Then the esophagus is retracted and fixed circumferentially in the Surgery, Full Professor of General pillars of the diaphragm with six or seven point. The goal is at least on the third part of the esophagus, to achieveResults: its broad mobilization and rectification of it; then is added a traditional Heller myotomy. Submission:Surgery At UNIRIO and PUC- Rio, Brazil Published: The mean dysphagia score in pre-op was 10points and in the post- op was 1.3 points (maximum October 09, 2019 of 10 points being observed each between the pre and postoperative 8.67 points, 86.7%) The mean October 24, 2019 hospitalization time was one day. -

Patient Education Guide
Patient Education Guide Guidance and support to help you manage your gastrostomy tube (g-tube) Balloon ® Mini ONE Buttons The Leader in Enteral Device Innovation Contents Balloon Mini ONE® Button 2 How the Balloon Mini ONE® Button is Different 17 Decompression (Venting the 4 Balloon Mini ONE® Button Kit Contents Stomach / Releasing Gas) 18 G-Tube Related Concerns 6 Attaching Feed Sets 22 Stoma Site Related Concerns 8 Feeding with Your Mini ONE® Feeding Tube 23 Administering Medications 10 Stoma Site Care / Button Maintenance 24 Feeding Sets and Accessories 12 Replacing the Balloon Mini ONE® Button 25 Re-Ordering Information 16 Special Concerns for Children 1 Introduction to Tube Feeding Proper nutrition is essential to maintaining our bodies’ health, growth and ability to heal. Various medical conditions may make it difficult or impossible for a person to eat, and thus deny the body of essential nutrients. In such cases, a gastrostomy tube (g-tube) is inserted to provide direct access to the stomach for feeding. A g-tube is a convenient, comfortable and effective means for delivering nutritional formulas to the George J. Picha, body. These nutritional formulas are either commercially available or homemade using M.D., Ph.D., F.A.C.S. a food processor. A physician will prescribe the proper feeding procedure, formula Founder of AMT and Co-inventor of the and amount of water to most effectively feed each patient. first Button low profile feeding device. Comfort and Confidence are Important, Too AMT specializes in enteral devices, our As a caregiver or patient, we believe you have a right to the most comfortable core business. -

Content Production and Management by Applying Artificial Intelligence Techniques for Content Composition, Representation (Format) and Workflow; Ii
IST-2-511299 Automating Production of Cross Media Content for Multi-channel Distribution www.AXMEDIS.org CCoonntteenntt PPrroodduuccttiioonn Version: 1.0 URL: http://www.axmedis.org/ David Luigi FUSCHI GIUNTI Interactive Labs Laurence PEARCE XIM AXMEDIS: Automating Production of Cross Media Content for Multi-channel Distribution www.AXMEDIS.org 1 Preface Digital-content market is urging better pricing and value-for-money for industry products and services. This is clearly evident in the recent price reductions by major companies in the sector. The containment of sale prices is a vital key when setting up a viable and sustainable business venture in the digital cross media content. Possible solutions to this challenge could be found by automating, accelerating and re- structuring production processes, and providing solution to the content protection. Such solutions will en- able the production processes to be faster and cheaper, while at the same time providing new capabilities to support safer distribution. AXMEDIS aims to meet the challenges of market demand by: i. reducing costs for content production and management by applying Artificial Intelligence techniques for content composition, representation (format) and workflow; ii. reducing distribution and aggregation costs in order to increase accessibility with a Peer-to-Peer (P2P) platform at Business-to-Business (B2B) level, which can integrate content management sys- tems and workflows; iii. providing new methods and tools for innovative and flexible Digital Rights Management (DRM), -

CQ-TV Is Produced on an ATARI MEGA ST4 Computer System, Using the PROTEXT Word Processing Package and the TIMEWORKS Desktop Publishing Package
EQINIU No. 156 NOVEMBER 1991 BRITISH AMATEUR TELEVISION CLUB GUESS WHAT ? CONTENTS 7 SATELLITE TV NEWS Trevor Brown G8CJS 9 S-VHS/CVBS TO RGB CONVERTER H.Reelen 20 PULSAR - DIGITAL CIRCUIT SIMULATOR Mike Wooding G6IQM SOFTWARE PACKAGE REVIEW 25 CONSTRUCTING AERIAL PHASING LINES Ron Neyens NOCIH 26 MORE CROPREDY BOARD TIPS Brian Kelly GW6BWX 28 A NEW SSTV STANDARD ? Mike Wooding G6IQM 31 CONTEST CALENDAR 32 A 5-ELEMENT VIDEO FILTER John Cronk GW3MEO 33 IN THE STUDIO PART-13 John Goode 40 USING OSCILLOSCOPES Part-9 (Conclusion) Mike Wooding G6IQM SUP-1 to SUP-8 PUBLICATIONS AND MEMBERS' SERVICES SUPPLEMENT 45 THE DAYTON HAMFEST REPORT Andy Emmerson G8PTH 48 A DETACHABLE HIGH POWER E-PLANE PROBE Dr.J.A.Share Msc. Phd. 50 BEYOND TTL Part-1 Trevor Brown G8CJS 56 10GHZ AN THE EASY WAY Part-4 Jim Toon GOFNH 59 FEEDBACK - THE TDA3590 AGAIN Tom Mitchell G3LMX 62 BROADCAST BAND DX-TV RECEPTION Garry Smith & Keith Hamer 67 CONTEST NEWS Bob Platts G8OZP 68 NON THE AIR Andy Emmerson G8PTH 71 HOW TO GET INTO SAT N - WITH A MELON INSTEAD OF A DISH ! REVIEW Andy Emmerson G8PTH 73 THE BATC AT THE HAMFEST Brian Summers G8GQS 75 REPEATER GROUP AFFILLIATIONS 76 THE BATC BULLETIN BOARD SYSTEM 77 MORE ON THATVIDEO FILTER Peter Grannell G4TQB CLOSE FOR PRESS FOR THE NEXT ISSUE 20th DECEMBER 1991 CQ-TV is produced on an ATARI MEGA ST4 computer system, using the PROTEXT word processing package and the TIMEWORKS desktop publishing package. The camera- ready artwork is produced on an NEC PINWRITER P2200 24-pin dot-matrix printer. -

Spectrophotometric Determination of Thorium with the Trisodium Salt of 2-(2-Hydroxy-3,6-Disulfo-1-Naphthylazo)
Ames Laboratory ISC Technical Reports Ames Laboratory 6-1953 Spectrophotometric determination of thorium with the trisodium salt of 2-(2-hydroxy-3,6-disulfo-1-naphthylazo)- benzenearsonic acid and some properties of complexes involved Carol H. Byrd Iowa State College Charles V. Banks Iowa State College Follow this and additional works at: http://lib.dr.iastate.edu/ameslab_iscreports Part of the Chemistry Commons Recommended Citation Byrd, Carol H. and Banks, Charles V., "Spectrophotometric determination of thorium with the trisodium salt of 2-(2-hydroxy-3,6-disulfo-1-naphthylazo)-benzenearsonic acid and some properties of complexes involved" (1953). Ames Laboratory ISC Technical Reports. 62. http://lib.dr.iastate.edu/ameslab_iscreports/62 This Report is brought to you for free and open access by the Ames Laboratory at Iowa State University Digital Repository. It has been accepted for inclusion in Ames Laboratory ISC Technical Reports by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Spectrophotometric determination of thorium with the trisodium salt of 2-(2-hydroxy-3,6-disulfo-1-naphthylazo)-benzenearsonic acid and some properties of complexes involved Abstract The ods ium salt of 2-(2-hydroxy-3 3 6-disulfo-1- naphthylazo)-benzenearsonic acid, hereinafter referred to as thorin, has been extensively employed as a reagent for the spectrophotometric determination of thorium in recent years. No studies of the nature of the reaction between thorin and thorium have been reported. In this work, an examination of some properties of the complexes involved has been initiated. Keywords Ames Laboratory Disciplines Chemistry This report is available at Iowa State University Digital Repository: http://lib.dr.iastate.edu/ameslab_iscreports/62 TABLE OF CONTENTS Page I. -

Pancreaticogastrostomy
eCommons@AKU Section of General Surgery Department of Surgery October 2017 Pancreaticogastrostomy - an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy - experience from a tertiary care center of Pakistan Tabish Chawla Aga Khan University, [email protected] Hassaan Bari Aga Khan University Shahrukh Effendi Follow this and additional works at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_gen Part of the Surgery Commons Recommended Citation Chawla, T., Bari, H., Effendi, S. (2017). Pancreaticogastrostomy - an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy - experience from a tertiary care center of Pakistan. Journal of Pakistan Medical Association, 67(10), 1621-1624. Available at: https://ecommons.aku.edu/pakistan_fhs_mc_surg_gen/76 1621 CASE SERIES Pancreaticogastrostomy — an alternate for dealing with pancreatic remnant after pancreaticoduodenectomy — experience from a tertiary care center of Pakistan Tabish Chawla, Hassaan Bari, Shahrukh Effendi Abstract as part of PD. Therefore it was associated with high Whipple's pancreaticoduodenectomy has been refined morbidity and mortality resulting from high rates of over the years to be a safe operation though the leakage from pancreatic stump. morbidity rate still remains high (30-50%). Pancreatic Pancreatcogastrostomy is a repopularized technique fistula is the most important cause of mortality which has been described previously in literature. 3 This following pancreaticoduodenectomy. To prevent it, study was done to review the experience of PG being surgeons have used two anastomotic techniques: done as an alternate to PJ after PD. pancreaticojejunostomy and pancreaticogastrostomy. Recent studies found that pancreaticogastrostomy is Material and Methods associated with fewer overall complications than It is a case series collected at the Department of Surgery of pancreaticojejunostomy. -

High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider
NUTRITIONINFLAMMATORY ISSUES BOWEL IN GASTROENTEROLOGY, DISEASE: A PRACTICAL SERIES APPROACH, #105 SERIES #73 Carol Rees Parrish, M.S., R.D., Series Editor High Risk Percutaneous Endoscopic Gastrostomy Tubes: Issues to Consider Iris Vance Neeral Shah Percutaneous endoscopy gastrostomy (PEG) tubes are a valuable tool for providing long- term enteral nutrition or gastric decompression; certain circumstances that complicate PEG placement warrant novel approaches and merit review and discussion. Ascites and portal hypertension with varices have been associated with poorer outcomes. Bleeding is one of the most common serious complications affecting approximately 2.5% of all procedures. This article will review what evidence exists in these high risk scenarios and attempt to provide more clarity when considering these challenging clinical circumstances. INTRODUCTION ince the first Percutaneous Endoscopic has been found by multiple authors to portend a poor Gastrostomy tube was placed in 1979 (1), they prognosis in PEG placement (3,4, 5,6,7,8). This review Shave become an invaluable tool for providing will endeavor to provide more clarity when considering long-term enteral nutrition (EN) and are commonly used these challenging clinical circumstances. in patients with dysphagia following stroke, disabling motor neuron diseases such as multiple sclerosis and Ascites & Gastric Varices amyotrophic lateral sclerosis, and in those with head The presence of ascites is frequently viewed as a and neck cancer.They are also used for patients with relative, if not absolute, contraindication to PEG prolonged mechanical intubation, as well as gastric placement. Ascites adds technical difficulties and the decompression in those with severe gastroparesis, risk for potential complications (see Table 1). -
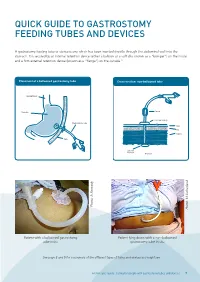
Quick Guide to Gastrostomy Feeding Tubes and Devices
QUICK GUIDE TO GASTROSTOMY FEEDING TUBES AND DEVICES A gastrostomy feeding tube or device is one which has been inserted directly through the abdominal wall into the stomach. It is secured by an internal retention device (either a balloon or a soft disc known as a “bumper”) on the inside and a firm external retention device (known as a “flange”) on the outside.11 Placement of a ballooned gastrostomy tube Cross-section: non-ballooned tube Oesophagus Stomach Clamp External Flange Gastrostomy tube Skin Fat Muscle Skin Internal Bumper Stomach Photo: APhoto: Kennedy Photo: MPhoto: Sutherland Patient with a ballooned gastrostomy Patient lying down with a non-ballooned tube insitu gastrostomy tube in situ See page 8 and 9 for a summary of the different types of tubes and devices you might see. A Clinician’s Guide: Caring for people with gastrostomy tubes and devices 7 Common features of gastrostomy feeding tubes and devices include, but are not limited to: Refer to manufacturer’s guidelines for advice on brand specific tube and device features Ballooned Gastrostomy Tube Ballooned Gastrostomy Tube With side port Without side port Feeding Port Feeding Port (Enteral Dispenser (Enteral Dispenser and Feed Bag and Feed Bag connect here) connect here) ml/cc Balloon Port Balloon Port ml/cc Side Port (X ml/cc) (X ml/cc) French (size) [For example:16/18/20] French (size) [For example:16/18/20] FR FR cm markings cm markings External External Flange Flange Balloon Balloon Non-ballooned Gastrostomy Tube Non-ballooned Gastrostomy Tube with collapsible internal -

Minionelow Profile Balloon G-Tube Patient Education Guide
Innovating. Educating. Changing Lives.™ ® MiniONELow Profile Balloon G-Tube Patient Education Guide Guidance & support to help you manage your gastrostomy tube (G-tube) Patient Education Guide Contents Introduction to Tube Feeding ® MiniONE Balloon Button Patient Education Guide Proper nutrition is essential to maintaining our bodies’ health, growth, and ability to heal. Introduction to Tube Feeding 1-3 Various medical conditions may make it difficult or impossible for a person to eat, and thus deny the body of essential nutrients. In such cases, a gastrostomy tube (G-tube) may be Enteral Nutrition 4-5 inserted to provide direct access to the stomach for feeding. A G-tube is a convenient, How the MiniONE® is Different 6-9 comfortable and effective means for delivering nutritional formulas to the body. These Attaching Feed Sets to the MiniONE® Balloon Button 10-11 nutritional formulas are either commercially available or homemade using a food processor. ® A healthcare provider will prescribe the proper feeding procedure, formula, and amount of Feeding With Your MiniONE 12-13 water to most effectively feed each patient. MiniONE® Maintenance 14-15 Replacing the MiniONE® Balloon Button 16-19 Comfort and Confidence are Important, Too As a caregiver or patient, we believe you have a right to the most comfortable and reliable Administering Medications 20 gastrostomy tubes, that’s why we developed the MiniONE® Balloon Button. Reading and Special Concerns For Children 21 understanding this guide, along with using our products, will help you achieve the comfort Device Related Concerns 22-25 and confidence you deserve. Stoma Site Related Concerns 26 Decompression 27 Compatible Feed Sets 28 Feed Set Accessories 29 Legacy Balloon Button Kit 30 MiniONE® Family 31 Re-Ordering information 32-33 Information Card 34-35 Share Your Story 36 Social Media 37 Customer Service 38 George J.