Brown Leaf Spot of Rice
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Overexpression of BSR1 Confers Broad-Spectrum Resistance Against Two Bacterial Diseases and Two Major Fungal Diseases in Rice
Breeding Science 66: 396–406 (2016) doi:10.1270/jsbbs.15157 Research Paper Overexpression of BSR1 confers broad-spectrum resistance against two bacterial diseases and two major fungal diseases in rice Satoru Maeda1), Nagao Hayashi1), Takahide Sasaya2,3) and Masaki Mori*1) 1) Institute of Agrobiological Sciences, NARO (NIAS), Tsukuba, Ibaraki 305-8602, Japan 2) NARO Agricultural Research Center (NARC), Tsukuba, Ibaraki 305-8666, Japan 3) Present address: NARO, Tsukuba, Ibaraki 305-8517, Japan Broad-spectrum disease resistance against two or more types of pathogen species is desirable for crop im- provement. In rice, Xanthomonas oryzae pv. oryzae (Xoo), the causal bacteria of rice leaf blight, and Magnaporthe oryzae, the fungal pathogen causing rice blast, are two of the most devastating pathogens. We identified the rice BROAD-SPECTRUM RESISTANCE 1 (BSR1) gene for a BIK1-like receptor-like cytoplas- mic kinase using the FOX hunting system, and demonstrated that BSR1-overexpressing (OX) rice showed strong resistance to the bacterial pathogen, Xoo and the fungal pathogen, M. oryzae. Here, we report that BSR1-OX rice showed extended resistance against two other different races of Xoo, and to at least one other race of M. oryzae. In addition, the rice showed resistance to another bacterial species, Burkholderia glumae, which causes bacterial seedling rot and bacterial grain rot, and to Cochliobolus miyabeanus, another fungal species causing brown spot. Furthermore, BSR1-OX rice showed slight resistance to rice stripe disease, a major viral disease caused by rice stripe virus. Thus, we demonstrated that BSR1-OX rice shows remarkable broad-spectrum resistance to at least two major bacterial species and two major fungal species, and slight resistance to one viral pathogen. -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -

Against the Brown Spot Pathogen Cochliobolus Miyabeanus
the brown the brown spot pathogen Silicon - induced induced resistance in rice ( / Magnum Photos Vink © John John © Cochliobolus Cochliobolus miyabeanus Oryza Oryza sativa L.) against Jonas Van Bockhaven Van Jonas Jonas Van Bockhaven Silicon-induced resistance in rice (Oryza sativa L.) against the brown spot pathogen Cochliobolus miyabeanus 2014 ISBN 978-90-5989-721-2 Silicon-induced resistance in rice (Oryza sativa L.) against the brown spot pathogen Cochliobolus miyabeanus Jonas VAN BOCKHAVEN Thesis submitted in fulfillment of the requirements for the degree of Doctor (PhD) in Applied Biological Sciences: Agricultural Sciences "We are all in the gutter, but some of us are looking at the stars" Oscar Wilde Promotor: Prof. Dr. ir. Monica H¨ofte Co-promotor: Dr. ir. David De Vleesschauwer Laboratory of Phytopathology Department of Crop Protection Ghent University Dean: Prof. Dr. Ir. Guido Van Huylenbroeck Rector: Prof. Dr. Anne De Paepe Dutch translation of the title: Silicium-ge¨ınduceerderesistentie tegen Cochliobolus miyabeanus in rijst (Oryza sativa L.) Cover illustration: The cover picture is made by John Vink (www.johnvink.com/JohnVinkSite), a Belgian photographer who is a member of the renowned photographic cooperative Magnum Photos. He took the cover picture in Myanmar showing rice fields and an elderly Palaung woman carrying rice stems to her house, some 3 kilometers away. I cropped the picture to make it fit the cover, the original picture is displayed below. There are several reasons why I chose this picture. First of all, I think this image is beautiful and one can not overestimate the importance of aesthetics. Second and more importantly, this picture represents the main motivations for starting my PhD project. -

Lecture-1 Diseases of Rice
LECTURE-1 DISEASES OF RICE 1.1 BLAST Causal organism: Pyricularia oryzae (Sub-division: Deuteromycotina) Perfect stage - Magnaporthe grisea Symptoms The fungus attacks the crop at all stages from seedlings in nursery to heading in main field. The typical symptoms appear on leaves, leaf sheath, rachis, nodes and even the glumes are also attacked. Leaf blast: On the leaves, the lesions start as small water soaked bluish green specks, soon enlarge and form characteristic spindle shaped spots with grey centre and dark brown margin. The spots join together as the disease progresses and large areas of the leaves dry up and wither. Node blast: In infected nodes, irregular black areas that encircle the nodes can be noticed. The affected nodes may break up and all the plant parts above the infected nodes may die (Node blast). Neck blast: At the flower emergence, the fungus attacks the peduncle which is engirdled, and the lesion turns to brownish-black. This stage of infection is commonly referred to as rotten neck/neck rot/neck blast/panicle blast. In early neck infection, grain filling does not occur and the panicle remains erect like a dead heart caused by a stem borer. In the late infection, partial grain filling occurs. Small brown to black spots also may be observed on glumes of the heavily infected panicles. Fig 1.1: Leaf Blast of Rice Fig 1.2: Node Blast of Rice 1 Disease Cycle Primary Infection – Mycelium & Conidia in the infected straw & seeds Secondary Infection – Conidia spread by wind and rain splashes. Favourable Condition Application of excessive doses of nitrogenous fertilizers, intermittent drizzles, cloudy weather, high relative humidity (93-99 per cent), low night temperature (between 15-20 0C or less than 26 0C). -

(Oryza Spp.) List of Descriptors
Descriptors for wild and cultivated Rice(Oryza spp.) List of Descriptors Allium (E,S) 2000 Peach * (E) 1985 Almond (revised) * (E) 1985 Pear * (E) 1983 Apple * (E) 1982 Pearl millet (E,F) 1993 Apricot * (E) 1984 Pepino (E) 2004 Avocado (E,S) 1995 Phaseolus acutifolius (E) 1985 Bambara groundnut (E,F) 2000 Phaseolus coccineus * (E) 1983 Banana (E,S,F) 1996 Phaseolus lunatus (P) 2001 Barley (E) 1994 Phaseolus vulgaris * (E,P) 1982 Beta (E) 1991 Pigeonpea (E) 1993 Black pepper (E,S) 1995 Pineapple (E) 1991 Brassica and Raphanus (E) 1990 Pistacia (excluding Pistacia vera) (E) 1998 Brassica campestris L. (E) 1987 Pistachio (E,F,A,R) 1997 Buckwheat (E) 1994 Plum * (E) 1985 Capsicum * (E,S) 1995 Potato variety * (E) 1985 Cardamom (E) 1994 Quinua * (S) 1981 Carrot (E,S,F) 1999 Rambutan (E) 2003 Cashew * (E) 1986 Rice * (E) 1980 Chenopodium pallidicaule (S) 2005 Rocket (E,I) 1999 Cherry * (E) 1985 Rye and Triticale * (E) 1985 Chickpea (E) 1993 Safflower * (E) 1983 Citrus (E,F,S) 1999 Sesame * (E) 2004 Coconut (E) 1992 Setaria italica and S. pumila (E) 1985 Coffee (E,S,F) 1996 Shea tree (E) 2006 Cotton * (Revised) (E) 1985 Sorghum (E,F) 1993 Cowpea * (E) 1983 Soyabean * (E,C) 1984 Cultivated potato * (E) 1977 Strawberry (E) 1986 Date palm (F) 2005 Sunflower * (E) 1985 Echinochloa millet * (E) 1983 Sweet potato (E,S,F) 1991 Eggplant (E,F) 1990 Taro (E,F,S) 1999 Faba bean * (E) 1985 Tea (E,S,F) 1997 Fig (E) 2003 Tomato (E, S, F) 1996 Finger millet * (E) 1985 Tropical fruit * (E) 1980 Forage grass * (E) 1985 Ulluco (S) 2003 Forage legumes * (E) 1984 Vigna aconitifolia and V. -

Diversity of Natural Products of the Genera Curvularia and Bipolaris
fungal biology reviews 33 (2019) 101e122 journal homepage: www.elsevier.com/locate/fbr Review Diversity of natural products of the genera Curvularia and Bipolaris Afra KHIRALLAa,b, Rosella SPINAb, Sahar SALIBAb, Dominique LAURAIN-MATTARb,* aBotany Department, Faculty of Sciences and Technologies, Shendi University, P.O. Box 142, Shendi, Sudan bUniversite de Lorraine, CNRS, L2CM, F-54000, Nancy, France article info abstract Article history: Covering from 1963 to 2017. Received 24 May 2018 This review provides a summary of some secondary metabolites isolated from the genera Accepted 17 September 2018 Curvularia and Bipolaris from 1963 to 2017. The study has a broad objective. First to afford an overview of the structural diversity of these genera, classifying them depending on their Keywords: chemical classes, highlighting individual examples of chemical structures. Also some in- Anti-malarial activity formation regarding their biological activities are presented. Several of the compounds re- Bipolaris ported here were isolated exclusively from endophytic and pathogenic strains in culture, Curvularia while few from other sources such as sea Anemone and fish. Some secondary metabolites Fungicidal activity of the genus Curvularia and Bipolaris revealed a fascinating biological activities included: Leishmanicidal activity anti-malarial, anti-biofouling, anti-larval, anti-inflammatory, anti-oxidant, anti-bacterial, Secondary metabolites anti-fungal, anti-cancer, leishmanicidal and phytotoxicity. Herein, we presented a bibliog- raphy of the researches accomplished on the natural products of Curvularia and Bipolaris, which could help in the future prospecting of novel or new analogues of active metabolites from these two genera. ª 2018 British Mycological Society. Published by Elsevier Ltd. All rights reserved. -

Brown Spot (Cochliobolus Miyabeanus) Factsheet for R Ice D
Brown Spot (Cochliobolus Miyabeanus) Causal agent: Fungus Geographical Distribution Management Strategies . This disease can occur in all rice 1. Cultural control growing areas of East Africa (refer to • Water stress can be prevented by Rice Production Areas Factsheet) application of alternative wetting-drying Fig 1. A spore of C. meyabeanus Crop damage and associated loss technique of irrigation. Source: Martin-Felix et al., 2017 Brown spot Roots of rice crop, infested • Infection by this fungus causes a • Soil nutrient stress can be avoided by serious damage on the leaves of both application of fertiliser at the appropriate Favourable conditions for disease young and adult rice plants, hence rates (see Water and Nutrient development reducing the photosynthetic area Management Factsheet). • Damaged seedlings have small, Fig 3. Brown lesions on rice • Inoculum reservoir can be destroyed by • The optimum conditions are: a leaf Source: Dr Lusike removing and/or burning of left-over temperature range of 16°C-36°C and a circular, yellow brown or brown lesions Wasilwa debris and any infected rice from the high relative humidity ranging 86-100% . that may girdle the coleoptile and distort field. • For infection to occur, the leaves must be primary and secondary leaves. • General crop health can be maintained wet for 8−24 hours. • Lesions on leaf sheaths are similar to by reducing rice-weed competition using • The disease is more common in fields those on the leaves. Infected glumes timely removal of weeds in the field. have water and nutrient deficiencies. and panicle branches have dark brown • Get rid of seedborne inoculum by using • Besides rice, the fungus attacks several to black oval spots. -

Stackburn, Seedling Blight, Leaf Spot of Rice -Alternaria Padwickii Alternaria Padwickii Is an Asexually Reproducing Fungus That Infects Seeds of Rice
U.S. Department of Agriculture, Agricultural Research Service Systematic Mycology and Microbiology Laboratory - Invasive Fungi Fact Sheets Stackburn, seedling blight, leaf spot of rice -Alternaria padwickii Alternaria padwickii is an asexually reproducing fungus that infects seeds of rice. It is one of several fungi responsible for seed discoloration, seed rot and seedling blight, but has also been detected as a sheath-rotting pathogen (Naeimi et al., 2003). It occurs in southern Asia and in countries on other continents worldwide, but its presence in mainland North America is not confirmed. Transport to and transmission in new areas may be prevented by use of tested clean seed. Where the pathogen is already present, application of seed treatments should reduce disease incidence, but the fungus has an undetermined ability to survive as sclerotia in plant debris and soil. Alternaria padwickii (Ganguly) M.B. Ellis 1971 (Ascomycetes, Pleosporales) Colonies on PDA spreading, grayish, sporulating. Reverse often deep pink or purple. Conidiophores solitary, unbranched, smooth, 100-180 x 3-4 µm. Apices often swollen to 5-6 µm, minutely echinulate, bearing one monotretic conidiogenous cell. Conidia single, fusiform to obclavate, with filamentous true beak, 95-170 (including beak) x 11-20 µm. Body hyaline to straw- or golden-brown, with 3-5, commonly 4, transverse septa, often constricted at septa, smooth or minutely echinulate. Beak hyaline, 0-1 or more septate, half to more than half the length of spore body. Sclerotia spherical, black, multicellular, walls reticulate, 50-200 µm diam. For further details, see Padwick (1950); Ellis (1971); Ellis and Holiday (1972); Jain (1975). -

Characterising Plant Pathogen Communities and Their Environmental Drivers at a National Scale
Lincoln University Digital Thesis Copyright Statement The digital copy of this thesis is protected by the Copyright Act 1994 (New Zealand). This thesis may be consulted by you, provided you comply with the provisions of the Act and the following conditions of use: you will use the copy only for the purposes of research or private study you will recognise the author's right to be identified as the author of the thesis and due acknowledgement will be made to the author where appropriate you will obtain the author's permission before publishing any material from the thesis. Characterising plant pathogen communities and their environmental drivers at a national scale A thesis submitted in partial fulfilment of the requirements for the Degree of Doctor of Philosophy at Lincoln University by Andreas Makiola Lincoln University, New Zealand 2019 General abstract Plant pathogens play a critical role for global food security, conservation of natural ecosystems and future resilience and sustainability of ecosystem services in general. Thus, it is crucial to understand the large-scale processes that shape plant pathogen communities. The recent drop in DNA sequencing costs offers, for the first time, the opportunity to study multiple plant pathogens simultaneously in their naturally occurring environment effectively at large scale. In this thesis, my aims were (1) to employ next-generation sequencing (NGS) based metabarcoding for the detection and identification of plant pathogens at the ecosystem scale in New Zealand, (2) to characterise plant pathogen communities, and (3) to determine the environmental drivers of these communities. First, I investigated the suitability of NGS for the detection, identification and quantification of plant pathogens using rust fungi as a model system. -

Cochliobolus Heterostrophus
May 20Pathogen of the month – May 2020 a b c 50 mm d Fig. (a) Germinating conidium with appressoria (red arrows); (b) formation of an extracellular matrix around the appressorium and early formation of subcuticular hyphae; (c) subcuticular hyphal growth from a stomate (red arrowheads) and; (d) hyphal growth within the leaf ,(1934) ,(1934) mesophyll layer. Maize leaf lesions infected with C. heterostrophus were hand cut and cleared overnight in glacial acetic acid: ethanol solution. They were rinsed in water and then treated with Calcofluor White stain in (a) or Lactophenol Blue solution in (b) ,(c) and (d). Images were acquired with an Olympus BX50 and a Nikon Eclipse Ti microscopes on the Bioimaging platform, La Trobe University. Common Name: Maydis leaf blight, southern leaf blotch Disease: Southern corn leaf blight (SCLB) or leaf spot; leaf blotch Classification: K: Fungi P: Ascomycota C: Dothideomycetes O: Pleosporales F: Pleosporaceae Cochliobolus heterostrophus (anamorph, Bipolaris maydis) is a necrotophic, hetrothallic fungus which infects the leaves of Drechsler maize. The species is subdivided into three races: race O, race T and race C. Race T is the most virulent to maize plants ) carrying the Texas cytoplasmic male sterile trait due to presence of approx. 1.2 Mb of DNA encoding genes for T-toxin production. Other members of the genus include the necrotrophic corn pathogen Cochliobolus carbonum, the oat pathogen, Cochliobolus victoriae, the rice pathogen, Cochliobolus miyabeanus, the sorghum pathogen, Bipolaris sorghicola, the sugarcane pathogen, Bipolaris sacchari, and the hemibiotrophic generalized cereal and grass pathogen, Cochliobolus sativus. All species mentioned above produce host selective toxins. -

Genetic Diversity for Sustainable Rice Blast Management in China: Adoption and Impact
Genetic diversity for sustainable rice blast management in China: Adoption and impact Promotor: Prof. dr. ir. H. van Keulen Hoogleraar bij de leerstoelgroep Plantaardige Productiesystemen, Wageningen Universiteit Co-promotor: Dr. ir. L. Bastiaans Universitair docent bij de leerstoelgroep Gewas- en Onkruidecologie, Wageningen Universiteit Promotiecommissie: Prof. dr. A. Kuyvenhoven (Wageningen Universiteit) Prof. dr. ir. C. Leeuwis (Wageningen Universiteit) Dr. S. Mohanty (International Rice Research Institute, Philippines) Dr. ir. G.J.T. Kessel (Plant Research International, Wageningen UR) Dit onderzoek is uitgevoerd binnen de C.T. de Wit Onderzoekschool: Production Ecology and Resource Conservation. Genetic diversity for sustainable rice blast management in China: Adoption and impact Imelda M. Revilla-Molina Proefschrift ter verkrijging van de graad van doctor op gezag van de rector magnificus van Wageningen Universiteit, Prof. dr. M.J. Kropff in het openbaar te verdedigen op dinsdag 6 januari 2009 des namiddags te half twee in de Aula Revilla-Molina, I.M. (2009) Genetic diversity for sustainable rice blast management in China: Adoption and impact PhD Thesis, Wageningen University, Wageningen, The Netherlands, 130 pp. With summaries in English, Dutch, and Chinese. ISBN: 978-90-8585-255-1 Abstract Revilla-Molina, I.M. (2009). Genetic diversity for sustainable rice blast management in China: Adoption and impact. PhD thesis. Wageningen University, Wageningen, The Netherlands. With summaries in English, Dutch and Chinese, 130 pp. The experience on rice blast in Yunnan Province, China, is one of the most successful and widely publicized examples of genetic diversification for disease suppression in practice. The wet, cool climate of the province is highly favourable for the development of rice blast epidemics. -
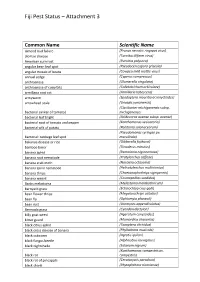
Fiji Pest Status – Attachment 3
Fiji Pest Status – Attachment 3 Common Name Scientific Name almond bud failure (Prunus necrotic ringspot virus) alomae disease (Taro bacilliform virus) American corn rust (Puccinia polysora) angular bean leaf spot (Pseudocercospora griseola) angular mosaic of beans (Cowpea mild mottle virus) annual sedge (Cyperus compressus) anthracnose (Glomerella cingulata) anthracnose of cucurbits (Colletotrichum orbiculare) armillaria root rot (Armillaria tabescens) armyworm (Spodoptera mauritia acronyctoides) arrowhead scale (Unaspis yanonensis) (Clavibacter michiganensis subsp. bacterial canker of tomato) michiganensis bacterial leaf blight (Acidovorax avenae subsp. avenae) bacterial spot of tomato and pepper (Xanthomonas vesicatoria) bacterial wilt of potato (Ralstonia solanacearum) (Pseudomonas syringae pv. bacterial: cabbage leaf spot maculicola) bakanae disease or rice (Gibberella fujikuroi) bamboo borer (Dinoderus minutus) banana aphid (Pentalonia nigronervosa) banana root nematode (Pratylenchus coffeae) banana scab moth (Nacoleia octasema) banana spiral nematode (Helicotylenchus multicinctus) banana thrips (Chaetanaphothrips signipennis) banana weevil (Cosmopolites sordidus) Banks melastoma (Melastoma malabathricum) barnyard grass (Echinochloa crus-galli) bean flower thrips (Megalurothrips usitatus) bean fly (Ophiomyia phaseoli) bean rust (Uromyces appendiculatus) Bermuda grass (Cynodon dactylon) billy goat weed (Ageratum conyzoides) bitter gourd (Momordica charantia) black citrus aphid (Toxoptera citricidus) black cross disease of banana (Phyllachora