From Predation to Management: Monitoring Wolf Distribution and Understanding Depredation Patterns from Attacks on Livestock
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Sicilian Wolf: Genetic Identity of a Recently Extinct Insular Population
bioRxiv preprint doi: https://doi.org/10.1101/453365; this version posted November 5, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. The Sicilian wolf: Genetic identity of a recently extinct insular population Angelici F.M.1*, Ciucani M.M. #2,3, Angelini S.4, Annesi F.5, Caniglia R6., Castiglia R.5, Fabbri E.6, Galaverni M.7, Palumbo D.8, Ravegnini G.4, Rossi L.8, Siracusa A.M.10, Cilli E.2 Affiliations: * Corresponding author # Co-first author: These authors equally contributed to the paper 1 FIZV, Via Marco Aurelio 2, I-00184 Roma, Italy 2 Laboratories of Physical Anthropology and Ancient DNA, Department of Cultural Heritage, University of Bologna, Ravenna, Italy; 3 Natural History Museum of Denmark, Copenhagen, Denmark 4 Dip.to Farmacia e Biotecnologia, Università di Bologna, Bologna, Italy 5 Dip.to Biologia e Biotecnologie ‘C. Darwin’, Sapienza Università di Roma, Roma, Italy 6 Area per la Genetica della Conservazione BIO-CGE, ISPRA, Ozzano dell’Emilia, Bologna, Italy 7 WWF Italia, Via Po 25/C, 00198 Roma, Italy 8 Museo di Ecologia di Cesena, Piazza Pietro Zangheri, 6, 47521 Cesena (FC), Italy 10 Dipartimento di Scienze Biologiche, Geologiche e Ambientali - Sez. Biologia Animale “Marcello La Greca”, Catania, Italy 1 bioRxiv preprint doi: https://doi.org/10.1101/453365; this version posted November 5, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. -

Avviso Pubblico Per La Presentazione Della
Ambito Territoriale Sociale di Larino Comuni associati di Larino (comune capofila), Bonefro, Casacalenda, Colletorto, Montelongo, Montorio nei Frentani, Morrone del Sannio, Provvidenti, Ripabottoni, Rotello, San Giuliano di Puglia, San Martino in Pensilis, Santa Croce di Magliano, Ururi AMBITO TERRITORIALE SOCIALE DI LARINO COMUNE CAPOFILA LARINO AVVISO PUBBLICO PER LA PRESENTAZIONE DELLA DOMANDA DI CONCESSIONE DEI TIROCINI DI INCLUSIONE SOCIALE PROMOSSI DALL'ATS DI LARINO (approvato con Determinazione del Responsabile Amministrativo dell'Ufficio di Piano n.947 del 23/10/2018) L'Ufficio di Piano dell'Ambito Territoriale Sociale di Larino (Convenzione per la gestione associata delle funzioni e dei servizi di ambito inerenti l’attuazione del Piano Sociale di Zona 2016 - 2018, ex art. 30 del D.Lgs. 267/2000) AVVISA che, in esecuzione della Deliberazione C.S. n. 11 del 18.10.2018 e del Regolamento d'ambito vigente in materia, è possibile presentare domanda di concessione dei Tirocini di inclusione sociale a valere sui fondi dell'Ambito Sociale di Larino. I tirocini di inclusione (codice regionale E8 - codice CISIS F3- Linee guida del 22.01.2015 approvate in conferenza di Stato -Regioni - D.G.R. n. 105 del 17.03.2016 - Regolamento d'ambito giusta Delib. C.S. n. 9 del 18.10.2018) si rivolgono alle persone prese in carico dal servizio sociale professionale competente che presentano bisogni complessi tali da richiedere interventi personalizzati di fuoriuscita dalla povertà. Pertanto, i tirocini si configurano come una misura di integrazione sociale e di sostegno al reddito, che si esplica in percorsi personalizzati di accompagnamento tesi all'inclusione sociale e lavorativa delle persone svantaggiate. -
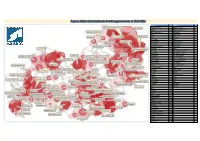
Cluster Molise
Regione Molise Casi Attualmente Positivi aggiornamento al: 28.03.2021 29/03/2021 60,00 Comune N° Comune N° Acquaviva d'Isernia 12 Petrella Tifernina 4 San Giacomo degli Schiavoni; 2 Termoli; 99 Agnone 51 Pettoranello del Molise 2 Baranello 10 Pietrabbondante 5 Montenero di Bisaccia; 3 Belmonte del Sannio 13 Pietracatella 1 Bojano 17 Portocannone 2 Bonefro 4 Pozzilli 2 Petacciato; 21 Campomarino; 29 Mafalda; 4 Busso 4 Provvidenti 3 Campobasso 105 Riccia 20 50,00 Portocannone; 2 Campodipietra 7 Rionero Sannitico 4 Guglionesi; 12 Campolieto 2 Ripalimosani 8 Campomarino 29 Roccamandolfi 1 Montecilfone; 1 Cantalupo nel Sannio 5 Roccavivara 2 Capracotta 8 Rotello 2 Capracotta; 8 San Martino in Pensilis; 19 Carpinone 2 San Giacomo degli Schiavoni 2 Casacalenda 16 San Giuliano di Puglia 6 Castelmauro 1 San Martino in Pensilis 19 Larino; 12 Trivento; 16 Castelpetroso 6 San Pietro Avellana 6 40,00 San Pietro Avellana; 6 Roccavivara; 2 Castelmauro; 1 Castelpizzuto 1 San Polo Matese 1 Castropignano 3 Santa Croce di Magliano 27 Belmonte del Sannio; 13 Guardialfiera; 2 Ururi; 3 Cercemaggiore 20 Santa Maria del Molise 1 Civitacampomarano; 4 Casacalenda; 16 Cercepiccola 1 Sant'Agapito 3 Agnone; 51 Montorio nei Frentani; 2 Cerro al Volturno 1 Sant'Angelo Limosano 2 Rionero Sannitico; 4 Chiauci 3 Sant'Elia a Pianisi 1 Rotello; 2 Civitacampomarano 4 Sepino 10 Pietrabbondante; 5 Lucito; 1 Bonefro; 4 Montelongo; 3 Colletorto 9 Sesto Campano 1 Ferrazzano 8 Spinete 1 30,00 Vastogirardi; 7 Pescolanciano; 4 Sant'Angelo Limosano; 2 Filignano 2 Termoli 99 Chiauci; -

Comuni Di Castropignano, Fossalto, Pietracupa E Torella Del Sannio
COMUNI DI CASTROPIGNANO, FOSSALTO, PIETRACUPA E TORELLA DEL SANNIO Provincia di Campobasso PROCEDURA APERTA PER LA FORNITURA DI ATTREZZATURE PER LA RACCOLTA DIFFERENZIATA E LA RIDUZIONE DEI RIFIUTI URBANI DISCIPLINARE DI GARA 1di16 AMMINISTRAZIONE AGGIUDICATRICE Regione Molise – Servizio centrale Unica di Committenza sede in Campobasso alla via XXIV maggio,130 – telef. 0874 – 429810 – pec: [email protected] profilo del committente www.regione.molise.it AMMINISTRAZIONE CONTRAENTE COMUNE DI CASTROPIGNANO Via Marconi, 1 - 86010 CASTROPIGNANO (CB) Telefono: 0874-503132 – Fax: 0874-503522 Indirizzo mail: [email protected] PEC: [email protected] OGGETTO DELLA FORNITURA La procedura è finalizzata all'acquisto di attrezzature e mezzi innovativi finalizzati all'applicazione della tariffa puntuale e alla tracciabilità del rifiuto. In particolare: - N. 5 SISTEMI DI RILEVAMENTO PORTATILI per la raccolta dei rifiuti - N. 1 PORTALE E SOFTWARE/SERVER - N. 1 CASETTA INFORMATIZZATA - N. 1 MEZZO DI RACCOLTA CON VASCA DA 2,2 MC - N. 1 MEZZO DI RACCOLTA CON VASCA DA 5-6 MC - N. 1 MEZZO DI RACCOLTA CON VASCA DA 7-8 MC Le specifiche tecniche della fornitura sono riportate nel Capitolato Speciale d’appalto e relative schede tecniche. IMPORTO DELLA FORNITURA L’importo totale della fornitura è indicato in € 163.934,43 (Euro centosessantatremilanovecentotrentaquattro/43 - I.V.A. eclusa). Il prezzo è da considerarsi comprensivo di ogni onere che il Disciplinare e il Capitolato di gara individuano a carico del fornitore: trasporto e consegna nel luogo che sarà indicato dalla stazione appaltante, oneri relativi a eventuali pratiche di immatricolazione e omologazione, ecc. Lo stesso sarà considerato fisso e invariabile, indipendentemente da qualsiasi eventualità avesse a verificarsi. -

Comune Di San Biase - Protocollo in Arrivo N
Provincia Denominazione Istituzione Scolastica di Riferimento Denominazione Plesso Comune Campobasso IST. SUPERIORE "MARIO PAGANO" L.CLASSICO "M.PAGANO" CAMPOBASSO Campobasso IST. SUPERIORE "MARIO PAGANO" L. SCIENTIFICO - RICCIA RICCIA Campobasso IST. SUPERIORE "MARIO PAGANO" L.ARTISTICO "G.MANZU'" CAMPOBASSO Campobasso ISTITUTO OMNICOMPRENSIVO STATALE CASACALENDA I.MAGISTRALE - CASACALENDA CASACALENDA Campobasso ISTITUTO OMNICOMPRENSIVO STATALE CASACALENDA I.PROF.I.A.MAN.ASS.TECNICA- CASACALENDA CASACALENDA Campobasso ISTITUTO OMNICOMPRENSIVO STATALE CASACALENDA IST.TECN.ECON.F.MARKET. - CASACALENDA CASACALENDA Campobasso ISTITUTO SUPERIORE BOJANO L.S. - L.S.U. - L.S.A. -BOIANO BOIANO Campobasso ISTITUTO SUPERIORE BOJANO I.P.S.E.O.A. - MATESE VINCHIATURO Campobasso ISTITUTO SUPERIORE BOJANO ISTITUTO TECNICO ECONOMICO - BOIANO BOIANO Campobasso G.BOCCARDI IST. TEC. COMMERCIALE "G.BOCCARDI" TERMOLI Campobasso G.BOCCARDI I.T.COMMERCIALE "BOCCARDI" CORSO SERALE TERMOLI Campobasso G.BOCCARDI IST. TEC. NAUTICO E GEOM. "UGO TIBERIO" TERMOLI Campobasso L. PILLA IST. PROF. SERV. AGRICOLTURA E SVIL.RUR. CAMPOBASSO Campobasso L. PILLA ISTITUTO TECNICO ECONOMICO " L. PILLA" CAMPOBASSO Campobasso L. PILLA IST. TEC. COSTR. AMBIENTE E TERRITTORIO CAMPOBASSO Campobasso IISS ALFANO DA TERMOLI L.CLASSICO "G.PERROTTA" TERMOLI Campobasso IISS ALFANO DA TERMOLI LS LICEO SCIENT."ALFANO DA TERMOLI" TERMOLI Campobasso E. MAJORANA LICEO SCIENTIFICO-OPZ. SCIENZE APPLICATE TERMOLI Campobasso E. MAJORANA L.ARTISTICO "B.JACOVITTI" TERMOLI Campobasso E. MAJORANA I.T. INDUSTRIALE - E. MAJORANA TERMOLI Campobasso ISTITUTO SUPERIORE LARINO L.CLASSICO F.D OVIDIO LARINO Campobasso ISTITUTO SUPERIORE LARINO I.P.SERV. COMM.LI - CAMPOMARINO CAMPOMARINO Campobasso ISTITUTO SUPERIORE LARINO I. T. A E G. - I.TEC.AGR. E GEO. LARINO LARINO Campobasso ISTITUTO SUPERIORE LARINO IST. TECNICO "S.PARDO" CORSO SERALE LARINO Campobasso ISTITUTO SUPERIORE LARINO SEDE CARCERARIA AGGREGATA A I.T.A.E G. -

Appendice Statistica
POR MOLISE V ALUTAZIONE E X –ANTE A MBIENTALE APPENDICE STATISTICA POR MOLISE V ALUTAZIONE E X –ANTE A MBIENTALE Appendice Statistica INDICE ALLEGATI COMPARTO ARIA FIG.1.1 –Mappa del vento ............................................................................................... 1 FIG.1.2 – Emissioni di biossido di zolfo nella regione Molise ....................................... 2 FIG.1.3 – Emissioni di biossido di azoto nella regione Molise ....................................... 3 FIG.1.4 – Emissioni di composti organici volatici non metanici nella regione Molise... 4 FIG.1.5 – Emissioni di monossido di carbonio nella regione Molise.............................. 5 FIG.1.6 – Emissioni di particolato sospeso totale nella regione Molise.......................... 6 TAB.1.7a – Valori di immissione per la provincia di Campobasso ................................ 7 TAB.1.7 b – Valori di immissione per la provincia di Isernia....................................... 15 TAB.1.8 – Valori di immissione per la città di Campobasso ........................................ 22 FIG.1.9–Concentrazione medie giornaliere di Polveri Totali in Via Mazzini a CB...... 24 FIG.1.10 – Concentrazioni medie giornaliere di SO2 a Termoli................................... 24 FIG.1.11 – Concentrazioni medie giornaliere di Polveri Totali a Venafro ................... 25 TAB.1.12 – Aziende dei nuclei industriali Campobasso-Bojano, Isernia –Venafro, Termoli.......................................................................................................................... -
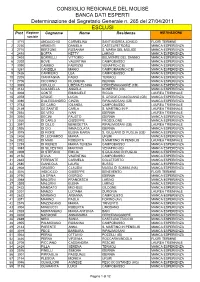
Elenco Idonei Ed Esclusi Progetto Formez
CONSIGLIO REGIONALE DEL MOLISE BANCA DATI ESPERTI Determinazione del Segretario Generale n. 265 del 27/04/2011 ESCLUSI Prot Ragione Cognome Nome Residenza MOTIVAZIONE sociale 1 3715 ANDACCHIO CARMELINA SANT'ANDREA JONICO FUORI TERMINE 2 2230 ARMENTI DANIELA CASTELPETROSO MANCA ESPERIENZA 3 2710 BERTONE ROSANNA S. MARIA DEL MOLISE MANCA ESPERIENZA 4 2493 BOFFA KETTY LARINO MANCA ESPERIENZA 5 2403 BORRELLI VITTORIO BELMONTE DEL SANNIO MANCA ESPERIENZA 6 2202 BOVE VALENTINA CAMPOBASSO MANCA ESPERIENZA 7 3090 CAMBIO FABRIZIO VENAFRO (CB) MANCA ESPERIENZA 8 3261 CANDELA MARIO CAMPOMARINO (CB) MANCA ESPERIENZA 9 2426 CARRIERO LEA CAMPOBASSO MANCA ESPERIENZA 10 2205 CIANFAGNA FABIO TERMOLI MANCA ESPERIENZA 11 2706 CICCHINO FILOMENA ISERNIA MANCA ESPERIENZA 12 3065 CIPULLO LIBERA ELSINA CERCEMAGGIORE (CB) MANCA ESPERIENZA 13 3144 COLABELLA ANGELA BONEFRO (CB) MANCA ESPERIENZA 14 3008 CONTE EMANUELE RICCIA LAUREA TRIENNALE 15 3078 CROCE LUCIA S. CROCE DI MAGLIANO (CB) MANCA ESPERIENZA 16 3086 D'ALESSANDRO CINZIA RIPALIMOSANI (CB) MANCA ESPERIENZA 17 2783 DE CARO IOLANDA CAMPOBASSO LAUREA TRIENNALE 18 2149 DE SANTIS CARLA S. MARTINO IN P. LAUREA TRIENNALE 19 2554 DE VITO IVAN ISERNIA LAUREA TRIENNALE 20 3050 DECINI FAUSTO ISERNIA MANCA ESPERIENZA 21 2920 DI CARLO GIUSEPPE FROSOLONE MANCA ESPERIENZA 22 3091 DI CILLO BENEDETTA RIPALIMOSANI (CB) LAUREA TRIENNALE 23 2926 DI CIO IMMACOLATA ISERNIA MANCA ESPERIENZA 24 3075 DI FIORE ELENA MARIA S. GIULIANO DI PUGLIA (CB) MANCA ESPERIENZA 25 2408 DI LEONARDO MARIA URURI MANCA ESPERIENZA 26 2932 DI MAIO -

The Reconstruction of San Giuliano Di Puglia After the October 31St 2002 Earthquake
13th World Conference on Earthquake Engineering Vancouver, B.C., Canada August 1-6, 2004 Paper No. 1805 THE RECONSTRUCTION OF SAN GIULIANO DI PUGLIA AFTER THE OCTOBER 31ST 2002 EARTHQUAKE M. INDIRLI1, P. CLEMENTE2, B. SPADONI3 SUMMARY This paper summarizes the activities in the emergency stages, post-emergency phase and reconstruction planning of San Giuliano di Puglia, struck by the October 31st 2002, earthquake. A brief description of the event, the seismic hazard analysis and the vulnerability evaluation of buildings, are first reported. The main results of the study, carried out by a scientists team (“San Giuliano Technical Scientific Group”, SG- TSG), are discussed. These activities concerned participation in the detailed evaluation of damage, draft of the demolition plan, ensuring safe conditions to the buildings to be repaired, actions for allowing residents to safely re-enter their non-damaged houses, and preparation of the reconstruction plan. In agreement with local people, the town will be reconstructed mostly where it was. The seismic safety will be ensured by adequate construction methods and possibly large use of Modern Antiseismic Techniques. INTRODUCTION October 31st, 2001, 11:35 local time: a moderate earthquake struck Molise Region (Italy), where the first shock (5.4 magnitude) was followed by another (5.3 magnitude) the day after. Major damage was evident in San Giuliano di Puglia, a small town located about 5 km from the epicenter (Figs. 1-2), completely evacuated after the seismic event, made inaccessible and protected by the police. The images of the primary school collapse, where twenty-seven children and one teacher died, went around the world. -

MOLISE - DIREZIONE GENERALE PER LA SALUTE Servizio Prevenzione, Veterinaria E Sicurezza Alimentare Ufficio Sicurezza Alimentare
REGIONE MOLISE - DIREZIONE GENERALE PER LA SALUTE Servizio Prevenzione, Veterinaria e Sicurezza Alimentare Ufficio Sicurezza Alimentare ELENCO DEGLI ALLEVATORI CHE COMPRANO IL MANGIME DA SOMMINISTRARE AGLI ANIMALI E CHE SVOLGONO ATTIVITA' DI DEPOSITO E STOCCAGGIO (art. 5, comma 1 Reg. (CE) n. 183/2005) ASREM - SEDE OPERATIVA DI AGNONE N. Cod_Aziendale Nome e Cognome o Ragione Sociale SEDE CODIFICA 1 002IS012 DE SIMONE VANDALINA AGNONE 4 2 002IS022 DI SABATO ROCCO AGNONE 4 3 002IS030 MARCOVECCHIO GELSUMINA AGNONE 4 4 002IS043 CELLILLI FILOMENA AGNONE 4 5 002IS044 CELLILLI FLORINDO AGNONE 4-6 6 002IS053 DI PIETRO GIANLUCA AGNONE 4 7 002IS059 GUALDIERI GIUSEPPINA AGNONE 4 8 002IS061 DI MARIO CARMINE AGNONE 4 9 002IS063 D'AGNILLO LUCIA AGNONE 4 10 002IS066 LONGHI GIOVINA AGNONE 4 11 002IS068 DI PINTO ANNAMARIA AGNONE 4 12 002IS070 LONGO IDA AGNONE 4 13 002IS079 PANNUNZIO GINO AGNONE 4-6 14 002IS081 PANNUNZIO NICOLA AGNONE 4-6 15 002IS082 PANNUNZIO TONINO AGNONE 4-6 16 002IS084 PANNUNZIO GENUINO AGNONE 4-6 17 002IS086 MARCOVECCHIO RACHELINA AGNONE 4 18 002IS087 PANNUNZIO ANGIOLINA AGNONE 4-6 19 002IS090 PALLOTTO DILIA AGNONE 4 20 002IS091 BUOSCIO GIUSEPPE AGNONE 4-6 21 002IS092 DI MENNA BINA AGNONE 4 22 002IS095 DIANA ERCOLINO AGNONE 4 23 002IS096 DIANA MICHELE AGNONE 4-6 24 002IS098 DIANA SANDRA AGNONE 4 25 002IS099 LAURIENTE BRUNO AGNONE 4 26 002IS100 ORLANDO ROCCO AGNONE 4 27 002IS101 ORLANDO ANGELO AGNONE 4-6 28 002IS103 ORLANDO CESARE AGNONE 4 29 002IS105 ORLANDO GIUSEPPE AGNONE 4 30 002IS106 ORLANDO MERCEDE AGNONE 4 31 002IS107 ORLANDO -

How Many Wolves Are There Currently in Italy?
Mamm Res DOI 10.1007/s13364-015-0247-8 ORIGINAL PAPER One, no one, or one hundred thousand: how many wolves are there currently in Italy? Marco Galaverni1 & Romolo Caniglia 1 & Elena Fabbri1 & Pietro Milanesi1 & Ettore Randi1,2 Received: 4 June 2015 /Accepted: 1 September 2015 # Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland 2015 Abstract Large carnivores in Italy and other European coun- trend of the population for each of the two management units: tries are protected by law to ensure their long-term conserva- Alps and Apennines. Results showed the occurrence of ap- tion. Estimates of abundance and demographic trends of their proximately 321 wolf packs in Italy, corresponding to 1269– populations are crucial for implementing effective conserva- 1800 wolves, possibly still underestimated. The Apennine tion and management strategies. However, it is challenging to sub-population seems to be almost the double in size (with obtain basic demographic parameters for elusive species such ca. 1212–1711 wolves in the period 2009–2013) compared to as the wolf (Canis lupus). Monitoring wolf populations by previous estimates (600–800 wolves between 2006 and 2011). standard field methods or non-invasive genetic approaches The Alpine sub-population, despite its ongoing eastwards ex- requires huge human efforts and may be exceedingly expen- pansion, appears rather stable (with 57–89 wolves). Overall, sive on a nation-wide scale. Aiming to obtain a first approxi- the current wolf population size and trends seem favorable, mate estimate of wolf distribution and abundance in Italy, we although the species is still locally threatened by widespread developed a systematic review procedure to analyze published poaching and accidents. -

Diapositiva 1
Le attività della Regione Molise nel Progetto ENERWOOD Metodologie per lo studio del potenziale da biomasse della Regione Molise Gruppo di lavoro progetto ENERWOOD Regione Molise N. Colonna ENEA A. Occhionero, R. Petti TASK FORCE AUTORITÀ AMBIENTALE P. De Pari, P. Gioia GEOSERVIZI srl Programmazione Energetica e Ruolo delle Biomasse Centrum Palace Hotel Campobasso, 4 dicembre 2007 Organizzazione della presentazione Scopi e metodologia Organizzazione database e risultati preliminari Elaborazioni cartografiche e analisi GIS 2 La stima delle biomasse • Le biomasse sono per loro natura disperse sul territorio. • Per “sfruttare” le biomasse è necessario sapere non tanto, o non solo, quante sono ma dove esse sono. • Qualsiasi impiego non può prescindere dalla fase di raccolta e concentrazione. • Il processo di trasformazione più idoneo dipende dalle caratteristiche della biomassa Umidità, Rapporto C/N, PCI 3 Quali biomasse? Residuali dal settore agricolo, Biomasse di provenienza forestale, Biomasse residuali dal settore agro-industriale. Classificate per origine e/o tipologia e/o caratteristiche fisico- chimiche 4 Obiettivo del lavoro • Quante • Dove • Tipo ……..meglio: Quante x Dove x Tipo Livello minimo di restituzione: comunale Disporre di questa informazione costituisce la base per valutare le opzioni di politica energetica, consente di valutare progetti presentati a livello regionale, permette di pianificare azioni o definire politiche mirate, valutare costi. 5 Quante biomasse ? Molise, su base annua migliaia di tonnellate CAMPOBASSO -

COMUNE DI RIPALIMOSANI (Provincia Di Campobasso)
COMUNE DI RIPALIMOSANI (provincia di Campobasso) Viale Marconi, 4 – 86025 RIPALIMOSANI – Tel. 0874-390959 – Fax 0874-39736 www.comune.ripalimosani.cb.it – email [email protected] – PEC [email protected] UFFICIO TECNICO – AREA TECNICA MANUTENTIVA E LAVORI PUBBLICI CENTRALE UNICA DI COMMITTENZA DEI COMUNI DI RIPALIMOSANI, MONTAGANO, MATRICE, CAMPOLIETO E CASTROPIGNANO Prot. 7092 Ripalimosani, 24/08/2019 Comune di Ripalimosani. Procedura negoziata per l’affidamento del “Servizio di trasporto alunni residenti fuori dal centro abitato di Ripalimosani” - CIG: ZBF2988E88. Avviso di manifestazione di interesse La Centrale Unica di Committenza dei Comuni di Ripalimosani, Montagano, Matrice, Campolieto e Castropignano presso il Comune di Ripalimosani, Viale Marconi, 4 – 86025 Ripalimosani – Tel. 0874- 390959 – Fax 0874-39736, pec: [email protected], indirà una procedura negoziata (art. 36, comma 2, lettera b) del D.lgs. 50/2016), per l’affidamento del “Servizio di trasporto alunni residenti fuori dal centro abitato di Ripalimosani” - CIG: ZBF2988E88. Importo complessivo servizi a base di gara € 39.500,00 di cui € 2.5000,00 per oneri della sicurezza non soggetti a ribasso d’asta. Aggiudicazione: “criterio del prezzo più basso”. Durata: dal 01/10/2019 al 30/06/2020 (9 mesi) con esclusione delle giornate di chiusura della scuola. Termine per la ricezione della manifestazione di interesse: 14/09/2019 ore 12,00. Allegato al presente avviso il capitolato/disciplinare d’appalto. Ai sensi della legge n. 241/1990 si comunica che il Responsabile del Procedimento di gara è l’Ing. Vincenzo Picciano, Responsabile dell’Area Tecnica Manutentiva e Lavori Pubblici, contattabile al n. 0874-390959. Per informazioni e chiarimenti è possibile contattare il Geom.