GILENYA® Cardiac Arrhythmias Requiring Anti-Arrhythmic Treatment with Class Ia Or Safely and Effectively
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Change of Fingolimod Patient Profiles Over Time
Journal of Personalized Medicine Article The Change of Fingolimod Patient Profiles over Time: A Descriptive Analysis of Two Non-Interventional Studies PANGAEA and PANGAEA 2.0 Tjalf Ziemssen 1,* and Ulf Schulze-Topphoff 2 1 Zentrum für Klinische Neurowissenschaften, Universitätsklinikum Carl Gustav Carus, D-01307 Dresden, Germany 2 Novartis Pharma GmbH, D-90429 Nuremberg, Germany; [email protected] * Correspondence: [email protected] Abstract: (1) Background: Fingolimod (Gilenya®) was the first oral treatment for patients with relapsing-remitting multiple sclerosis (RRMS). Since its approval, the treatment landscape has changed enormously. (2) Methods: Data of PANGAEA and PANGAEA 2.0, two German real-world studies, were descriptively analysed for possible evolution of patient profiles and treatment behavior. Both are prospective, multi-center, non-interventional, long-term studies on fingolimod use in RRMS in real life. Data of 4229 PANGAEA patients (recruited 2011–2013) and 2441 PANGAEA 2.0 patients (recruited 2015–2018) were available. Baseline data included demographics, RRMS characteristics and disease severity. (3) Results: The mean age of PANGAEA and PANGAEA 2.0 patients was similar (38.8 vs. 39.2 years). Patients in PANGAEA 2.0 had shorter disease duration (7.1 vs. 8.2 years) and fewer relapses in the year before baseline (1.2 vs. 1.6). Disease severity at baseline estimated by Citation: Ziemssen, T.; EDSS and SDMT was lower in PANGAEA 2.0 patients compared to PANGAEA (EDSS difference Schulze-Topphoff, U. The Change of 1.0 points; SDMT difference 3.3 points). (4) Conclusions: The results hint at an influence of changes in Fingolimod Patient Profiles over the treatment guidelines and the label on fingolimod patients profiles over time. -

Librarianship and the Philosophy of Information
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Library Philosophy and Practice (e-journal) Libraries at University of Nebraska-Lincoln July 2005 Librarianship and the Philosophy of Information Ken R. Herold Hamilton College Follow this and additional works at: https://digitalcommons.unl.edu/libphilprac Part of the Library and Information Science Commons Herold, Ken R., "Librarianship and the Philosophy of Information" (2005). Library Philosophy and Practice (e-journal). 27. https://digitalcommons.unl.edu/libphilprac/27 Library Philosophy and Practice Vol. 3, No. 2 (Spring 2001) (www.uidaho.edu/~mbolin/lppv3n2.htm) ISSN 1522-0222 Librarianship and the Philosophy of Information Ken R. Herold Systems Manager Burke Library Hamilton College Clinton, NY 13323 “My purpose is to tell of bodies which have been transformed into shapes of a different kind.” Ovid, Metamorphoses Part I. Library Philosophy Provocation Information seems to be ubiquitous, diaphanous, a-categorical, discrete, a- dimensional, and knowing. · Ubiquitous. Information is ever-present and pervasive in our technology and beyond in our thinking about the world, appearing to be a generic ‘thing’ arising from all of our contacts with each other and our environment, whether thought of in terms of communication or cognition. For librarians information is a universal concept, at its greatest extent total in content and comprehensive in scope, even though we may not agree that all information is library information. · Diaphanous. Due to its virtuality, the manner in which information has the capacity to make an effect, information is freedom. In many aspects it exhibits a transparent quality, a window-like clarity as between source and patron in an ideal interface or a perfect exchange without bias. -

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-527 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number 22-527 Priority or Standard P Submit Date 12-21-2009 Received Date 12-21-2009 PDUFA Goal Date 9-21-2010 Division / Office FDA/ODE1 Reviewer Name Heather D. Fitter Review Completion Date August 26, 2010 Established Name Fingolimod (Proposed) Trade Name Gilenya Therapeutic Class S1P receptor modulator Applicant Novartis Formulation(s) Oral tablets Dosing Regimen 0.5 mg Indication(s) To reduce the frequency of relapses and delay the progression of disability in relapsing MS Intended Population(s) Relapsing Multiple Sclerosis Template Version: March 6, 2009 Clinical Review Heather D. Fitter, M.D. NDA 22-527 Fingolimod Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT......................................... 7 1.1 Recommendation on Regulatory Action ............................................................. 7 1.2 Risk Benefit Assessment.................................................................................... 8 2 INTRODUCTION AND REGULATORY BACKGROUND ........................................ 8 2.1 Product Information ............................................................................................ 9 2.2 Table of Currently Available Treatments for the Proposed Indication................. 9 2.3 Availability of Proposed Active Ingredient in the United States ........................ 12 2.4 Important Safety Issues with Consideration to Related Drugs......................... -
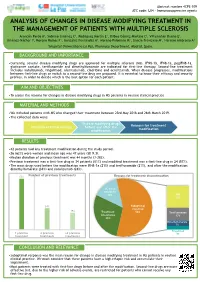
Analysis of Changes in Disease Modifying Treatment in The
Abstract number 4CPS-109 ATC code: L04 - Immunosuppressive agents ANALYSIS OF CHANGES IN DISEASE MODIFYING TREATMENT IN THE MANAGEMENT OF PATIENTS WITH MULTIPLE SCLEROSIS Arancón Pardo A1, Sobrino Jiménez C1, Rodríguez Martín E1, Bilbao Gómez-Martino C1, Villamañán Bueno E1, Jiménez-Nácher I1, Moreno Ramos F1, González Fernández A1, Moreno Palomino M1, García-Trevijano M1, Herrero Ambrosio A1 1Hospital Universitario La Paz, Pharmacy Department, Madrid, Spain. BACKGROUND AND IMPORTANCE •Currently, several disease modifying drugs are approved for multiple sclerosis (MS). IFNβ-1b, IFNβ-1a, pegIFNβ-1a, glatiramer acetate, teriflunomide and dimethylfumarate are indicated for first-line therapy. Second-line treatment includes natalizumab, fingolimod, alemtuzumab, cladribine and ocrelizumab. When disease progresses, modifications between first-line drugs or switch to a second-line drug are proposed. It is essential to know their efficacy and security profiles, in order to decide which is the best option for each patient. AIM AND OBJECTIVES •To assess the reasons for changes in disease modifying drugs in MS patients in routine clinical practice. MATERIAL AND METHODS •We included patients with MS who changed their treatment between 23rd May 2018 and 26th March 2019. •The collected data were: Disease modifying drugs Reasons for treatment Duration of initial therapy before and after the modification modification RESULTS •42 patients had any treatment modification during the study period. •26 (62%) were women and mean age was 47 years (SD 9.3). •Median duration of previous treatment was 44 months (3-282). •Previous treatment was a first-line drug in 34 patients (81%) and modified treatment was a first-line drug in 24 (57%). -

Patient Focused Disease State and Assistance Programs
Patient Focused Disease State and Assistance Programs Medication Medication Toll-free Brand (Generic) Website number Additional Resources Allergy/Asthma Xolair (omalizumab) xolair.com 1-866-4-XOLAIR lung.org Cardiovascular Pradaxa (dabigatran) pradaxa.com 877-481-5332 heart.org Praluent (alirocumab) praluent.com 844-PRALUENT thefhfoundation.org Repatha (evolocumab) repatha.com 844-REPATHA Tikosyn (dofetilide) tikosyn.com 800-879-3477 Crohn’s Disease Cimzia (certolizumab pegol) cimzia.com 866-4-CIMZIA crohnsandcolitis.com Humira (adalimumab) humira.com 800-4-HUMIRA crohnsforum.com Stelara (ustekinumab) stelarainfo.com 877-STELARA Dermatology Cosentyx (secukinumab) cosentyx.com 844-COSENTYX psoriasis.org Dupixent (dupilumab) dupixent.com 844-DUPIXENT nationaleczema.org Enbrel (etanercept) enbrel.com 888-4-ENBREL Humira (adalimumab) humira.com 800-4-HUMIRA Otezla (apremilast) otezla.com 844-4-OTEZLA Stelara (ustekinumab) stelarainfo.com 877-STELARA Taltz (ixekizumab) taltz.com 800-545-5979 Hematology Aranesp (darbepoetin alfa) aranesp.com 805-447-1000 chemocare.com Granix (filgrastim) granixrx.com 888-4-TEVARX hematology.org Jadenu (deferasirox) jadenu.com 888-282-7630 Neulasta (pegfilgrastim) neulasta.com 800-77-AMGEN Neupogen (filgrastim) neupogen.com 800-77-AMGEN Nivestym (filgrastim) nivestym.com 800-879-3477 Zarxio (filgrastim) zarxio.com 800-525-8747 Zytiga (abiraterone) zytiga.com 800-JANSSEN Hepatitis B Baraclude (entecavir) baraclude.com 800-321-1335 cdc.gov Viread (tenofovir disoproxil viread.com 800-GILEAD-5 hepb.org fumarate) -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -

Religious Fundamentalism in Eight Muslim‐
JOURNAL for the SCIENTIFIC STUDY of RELIGION Religious Fundamentalism in Eight Muslim-Majority Countries: Reconceptualization and Assessment MANSOOR MOADDEL STUART A. KARABENICK Department of Sociology Combined Program in Education and Psychology University of Maryland University of Michigan To capture the common features of diverse fundamentalist movements, overcome etymological variability, and assess predictors, religious fundamentalism is conceptualized as a set of beliefs about and attitudes toward religion, expressed in a disciplinarian deity, literalism, exclusivity, and intolerance. Evidence from representative samples of over 23,000 adults in Egypt, Iraq, Jordan, Lebanon, Pakistan, Saudi Arabia, Tunisia, and Turkey supports the conclusion that fundamentalism is stronger in countries where religious liberty is lower, religion less fractionalized, state structure less fragmented, regulation of religion greater, and the national context less globalized. Among individuals within countries, fundamentalism is linked to religiosity, confidence in religious institutions, belief in religious modernity, belief in conspiracies, xenophobia, fatalism, weaker liberal values, trust in family and friends, reliance on less diverse information sources, lower socioeconomic status, and membership in an ethnic majority or dominant religion/sect. We discuss implications of these findings for understanding fundamentalism and the need for further research. Keywords: fundamentalism, Islam, Christianity, Sunni, Shia, Muslim-majority countries. INTRODUCTION -

The Real-World Patient Experience of Fingolimod and Dimethyl Fumarate
Wicks et al. BMC Res Notes (2016) 9:434 DOI 10.1186/s13104-016-2243-8 BMC Research Notes RESEARCH ARTICLE Open Access The real‑world patient experience of fingolimod and dimethyl fumarate for multiple sclerosis Paul Wicks1* , Lawrence Rasouliyan2, Bo Katic1, Beenish Nafees3, Emuella Flood3 and Rahul Sasané4 Abstract Background: Oral disease-modifying therapies offer equivalent or superior efficacy and greater convenience versus injectable options. Objectives: To compare patient-reported experiences of fingolimod and dimethyl fumarate. Methods: Adult relapsing-remitting multiple sclerosis patients treated with fingolimod or dimethyl fumarate were recruited from an online patient community and completed an online survey about treatment side effects, discon- tinuation, and satisfaction. Results: 281 patients in four groups completed the survey: currently receiving fingolimod (CF, N 61), currently receiving dimethyl fumarate (CDMF, N 129), discontinued fingolimod (DF, N 32) and discontinued= dimethyl fuma- rate (DDMF, N 59). Reasons for treatment= switch were to take oral treatment =(CF: 63.3 %, CDMF: 61.8 %), side effects of prior medication= (CF: 67.3 %, CDMF: 44.1 %) and lack of effectiveness of prior medication (CF: 38.8 %, CDMF: 31.4 %). Main reasons for discontinuation were side effects (DF: 46.9 %, DDMF: 67.8 %) and lack of effectiveness (DF: 25.0 %, DDMF: 15.3 %). CDMF patients had an increased risk of abdominal pain, flushing, diarrhea, and nausea. Treatment satisfaction was highest among CF patients followed by CDMF, DF, and then DDMF patients. Conclusions: Discontinuation was driven by experience of side effects. Patients currently taking dimethyl fumarate were more likely to experience a side effect versus patients currently taking fingolimod. -

Fingolimod (Gilenya)
Fingolimod (Gilenya) This factsheet is about fingolimod, a Whether you’ll be offered this or any other DMT disease modifying therapy (DMT) for depends on whether you qualify for it based relapsing multiple sclerosis (MS). on guidelines used by your neurologist. These come from NICE and the Association of British At the end of this factsheet you’ll find out where Neurologists and are based on a drug’s Europe- you can get more information on this drug, other wide licence. drugs for MS and the benefits of early treatment. In England there are also rules from NHS England about who can have the different DMTs and when. This factsheet doesn’t cover everything about Scotland, Wales and Northern Ireland also have this drug and shouldn’t be used in place of their own guidelines for many DMTs. advice from your MS specialist team. For more information speak to them and read the online information from the drug’s makers (see the Whether you can have a drug also depends on section More information and support). if the NHS where you live will pay for it. NHS England guidelines on this tend to follow what What is fingolimod? NICE says. Fingolimod is a drug that was first given a licence These people can have fingolimod: to be used against relapsing MS in the UK in 2011. In England and Northern Ireland: In 2012 the National Institute for Health and Care Excellence (NICE) gave the go ahead for it to be • People with ‘highly active relapsing remitting used on the NHS. -

Peirce, Pragmatism, and the Right Way of Thinking
SANDIA REPORT SAND2011-5583 Unlimited Release Printed August 2011 Peirce, Pragmatism, and The Right Way of Thinking Philip L. Campbell Prepared by Sandia National Laboratories Albuquerque, New Mexico 87185 and Livermore, California 94550 Sandia National Laboratories is a multi-program laboratory managed and operated by Sandia Corporation, a wholly owned subsidiary of Lockheed Martin Corporation, for the U.S. Department of Energy’s National Nuclear Security Administration under Contract DE-AC04-94AL85000.. Approved for public release; further dissemination unlimited. Issued by Sandia National Laboratories, operated for the United States Department of Energy by Sandia Corporation. NOTICE: This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government, nor any agency thereof, nor any of their employees, nor any of their contractors, subcontractors, or their employees, make any warranty, express or implied, or assume any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represent that its use would not infringe privately owned rights. Reference herein to any specific commercial product, process, or service by trade name, trademark, manufacturer, or otherwise, does not necessarily con- stitute or imply its endorsement, recommendation, or favoring by the United States Government, any agency thereof, or any of their contractors or subcontractors. The views and opinions expressed herein do not necessarily state or reflect those of the United States Government, any agency thereof, or any of their contractors. Printed in the United States of America. This report has been reproduced directly from the best available copy. -

Citizen Science: Framing the Public, Information Exchange, and Communication in Crowdsourced Science
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2014 Citizen Science: Framing the Public, Information Exchange, and Communication in Crowdsourced Science Todd Ernest Suomela University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Communication Commons, and the Library and Information Science Commons Recommended Citation Suomela, Todd Ernest, "Citizen Science: Framing the Public, Information Exchange, and Communication in Crowdsourced Science. " PhD diss., University of Tennessee, 2014. https://trace.tennessee.edu/utk_graddiss/2864 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Todd Ernest Suomela entitled "Citizen Science: Framing the Public, Information Exchange, and Communication in Crowdsourced Science." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Doctor of Philosophy, with a major in Communication and Information. Suzie Allard, Major Professor We have read this dissertation and recommend its acceptance: Carol Tenopir, Mark Littmann, Harry Dahms Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) Citizen Science: Framing the Public, Information Exchange, and Communication in Crowdsourced Science ADissertationPresentedforthe Doctor of Philosophy Degree The University of Tennessee, Knoxville Todd Ernest Suomela August 2014 c by Todd Ernest Suomela, 2014 All Rights Reserved. -

The Theory of Everything
The Theory of Everything R. B. Laughlin* and David Pines†‡§ *Department of Physics, Stanford University, Stanford, CA 94305; †Institute for Complex Adaptive Matter, University of California Office of the President, Oakland, CA 94607; ‡Science and Technology Center for Superconductivity, University of Illinois, Urbana, IL 61801; and §Los Alamos Neutron Science Center Division, Los Alamos National Laboratory, Los Alamos, NM 87545 Contributed by David Pines, November 18, 1999 We discuss recent developments in our understanding of matter, we have learned why atoms have the size they do, why chemical broadly construed, and their implications for contemporary re- bonds have the length and strength they do, why solid matter has search in fundamental physics. the elastic properties it does, why some things are transparent while others reflect or absorb light (6). With a little more he Theory of Everything is a term for the ultimate theory of experimental input for guidance it is even possible to predict Tthe universe—a set of equations capable of describing all atomic conformations of small molecules, simple chemical re- phenomena that have been observed, or that will ever be action rates, structural phase transitions, ferromagnetism, and observed (1). It is the modern incarnation of the reductionist sometimes even superconducting transition temperatures (7). ideal of the ancient Greeks, an approach to the natural world that But the schemes for approximating are not first-principles has been fabulously successful in bettering the lot of mankind deductions but are rather art keyed to experiment, and thus tend and continues in many people’s minds to be the central paradigm to be the least reliable precisely when reliability is most needed, of physics.