The Change of Fingolimod Patient Profiles Over Time
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Anti-TNF Treatment in Inflammatory Bowel Disease
xx xx 48 ANNALSJanuary OF GASTROENTEROLOGY 20, 2007, KIFISIA, A THENS2007, 20(1):48-53x, G xxREECE x Lecture Anti-TNF Treatment in Inflammatory Bowel Disease Suna Yapali, Hulya over Hamzaoglu INTRODUCTION disease than ulcerative colitis.2 Moreover, enhanced secre- tion of TNF-alpha from lamina propria mononuclear cells Crohn’s disease and ulcerative colitis are chronic in- has been found in the intestinal mucosa of IBD patients.3 flammatory disorders of the gastrointestinal tract. Although In Crohn’s disease tissues, TNF-alpha positive cells have the primary etiological defect stil remains unknown, in the been found deeper in the lamina propria and in the submu- last decade impotant progress has been made concerning cosa, whereas TNF-alpha immunoreactivity in ulcerative the immunological basis of the disease. Genetic, environ- colitis is, mostly, located in subepithelial macrophages.4 mental and microbial factors result in repeated activation There may be insufficient increased release of soluble TNF of mucosal immune response. Tumor necrosis factor alpha receptor from lamina propria mononuclear cells of patients (TNF-alpha) is one of the central cytokines in the under- with IBD in response to enhanced secretion of TNF-alpha.5 lying pathogenesis of mucosal inflammation in inflamma- TNF-alpha in the stool have also been found from children tory bowel disease (IBD) and has been the primary target with active IBD,6 and elevated levels of TNF-alpha have of biologic therapies. been found increased in the serum of children with active 7 Role of TNF-alpha in pathogenesis of ulcerative colitis and Crohn’s colitis. inflammatory bowel disease Anti-TNF antibodies and fusion proteins: TNF-alpha is produced by activated macrophages and We certainly have many ways to block TNF-alpha. -

Biologics in the Treatment of Lupus Erythematosus: a Critical Literature Review
Hindawi BioMed Research International Volume 2019, Article ID 8142368, 17 pages https://doi.org/10.1155/2019/8142368 Review Article Biologics in the Treatment of Lupus Erythematosus: A Critical Literature Review Dominik Samotij and Adam Reich Department of Dermatology, University of Rzeszow, ul. Fryderyka Szopena 2, 35-055 Rzeszow, Poland Correspondence should be addressed to Adam Reich; adi [email protected] Received 7 May 2019; Accepted 18 June 2019; Published 18 July 2019 Academic Editor: Nobuo Kanazawa Copyright © 2019 Dominik Samotij and Adam Reich. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Systemic lupus erythematosus (SLE) is a chronic autoimmune infammatory disease afecting multiple organ systems that runs an unpredictable course and may present with a wide variety of clinical manifestations. Advances in treatment over the last decades, such as use of corticosteroids and conventional immunosuppressive drugs, have improved life expectancy of SLE suferers. Unfortunately, in many cases efective management of SLE is still related to severe drug-induced toxicity and contributes to organ function deterioration and infective complications, particularly among patients with refractory disease and/or lupus nephritis. Consequently, there is an unmet need for drugs with a better efcacy and safety profle. A range of diferent biologic agents have been proposed and subjected to clinical trials, particularly dedicated to this subset of patients whose disease is inadequately controlled by conventional treatment regimes. Unfortunately, most of these trials have given unsatisfactory results, with belimumab being the only targeted therapy approved for the treatment of SLE so far. -

Medical Review(S) Clinical Review
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 22-527 MEDICAL REVIEW(S) CLINICAL REVIEW Application Type NDA Application Number 22-527 Priority or Standard P Submit Date 12-21-2009 Received Date 12-21-2009 PDUFA Goal Date 9-21-2010 Division / Office FDA/ODE1 Reviewer Name Heather D. Fitter Review Completion Date August 26, 2010 Established Name Fingolimod (Proposed) Trade Name Gilenya Therapeutic Class S1P receptor modulator Applicant Novartis Formulation(s) Oral tablets Dosing Regimen 0.5 mg Indication(s) To reduce the frequency of relapses and delay the progression of disability in relapsing MS Intended Population(s) Relapsing Multiple Sclerosis Template Version: March 6, 2009 Clinical Review Heather D. Fitter, M.D. NDA 22-527 Fingolimod Table of Contents 1 RECOMMENDATIONS/RISK BENEFIT ASSESSMENT......................................... 7 1.1 Recommendation on Regulatory Action ............................................................. 7 1.2 Risk Benefit Assessment.................................................................................... 8 2 INTRODUCTION AND REGULATORY BACKGROUND ........................................ 8 2.1 Product Information ............................................................................................ 9 2.2 Table of Currently Available Treatments for the Proposed Indication................. 9 2.3 Availability of Proposed Active Ingredient in the United States ........................ 12 2.4 Important Safety Issues with Consideration to Related Drugs......................... -

Monoclonal Antibodies — a Revolutionary Therapy in Multiple Sclerosis
REVIEW ARTICLE Neurologia i Neurochirurgia Polska Polish Journal of Neurology and Neurosurgery 2020, Volume 54, no. 1, pages: 21–27 DOI: 10.5603/PJNNS.a2020.0008 Copyright © 2020 Polish Neurological Society ISSN 0028–3843 Monoclonal antibodies — a revolutionary therapy in multiple sclerosis Carmen Adella Sirbu1, 2, Magdalena Budisteanu1,3,4, Cristian Falup-Pecurariu5,6 1Titu Maiorescu University, Bucharest, Romania 2‘Dr. Carol Davila’ Central Military Emergency University Hospital, Clinic of Neurology, Bucharest, Romania 3‘Prof. Dr. Alex Obregia’ Clinical Hospital of Psychiatry, Psychiatry Research Laboratory, Bucharest, Romania 4‘Victor Babes’ National Institute of Pathology, Bucharest, Romania 5Faculty of Medicine, Transylvania University of Brașov, Brașov, Romania 6Department of Neurology, County Emergency Clinic Hospital, Brașov, Romania ABSTRACT Introduction. Multiple sclerosis (MS) has an increasing incidence and affects a young segment of the population, having a major impact on patients and consequently on society. The multifactorial aetiology and pathogenesis of this disease are incompletely known at present, but autoimmune aggression has a documented mechanism. State of the art. Since the 1990s, immunomodulatory drugs of high efficacy and a good safety profile have been launched. But the concept of NEDA (No Evidence of Disease Activity) remains the target to achieve. Thus, the new revolutionary class of monoclonal antibodies (moAbs) used in multiple medical fields, from this perspective represents a challenge even for multiple sclerosis, including the primary progressive form, for which there has been no treatment until recently. Clinical implications. In this article, we will review monoclonal antibodies’ use for MS, presenting their advantages and di- sadvantages, based on data accumulated since 2004 when the first monoclonal antibody was approved for active forms of the disease. -
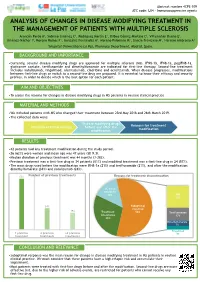
Analysis of Changes in Disease Modifying Treatment in The
Abstract number 4CPS-109 ATC code: L04 - Immunosuppressive agents ANALYSIS OF CHANGES IN DISEASE MODIFYING TREATMENT IN THE MANAGEMENT OF PATIENTS WITH MULTIPLE SCLEROSIS Arancón Pardo A1, Sobrino Jiménez C1, Rodríguez Martín E1, Bilbao Gómez-Martino C1, Villamañán Bueno E1, Jiménez-Nácher I1, Moreno Ramos F1, González Fernández A1, Moreno Palomino M1, García-Trevijano M1, Herrero Ambrosio A1 1Hospital Universitario La Paz, Pharmacy Department, Madrid, Spain. BACKGROUND AND IMPORTANCE •Currently, several disease modifying drugs are approved for multiple sclerosis (MS). IFNβ-1b, IFNβ-1a, pegIFNβ-1a, glatiramer acetate, teriflunomide and dimethylfumarate are indicated for first-line therapy. Second-line treatment includes natalizumab, fingolimod, alemtuzumab, cladribine and ocrelizumab. When disease progresses, modifications between first-line drugs or switch to a second-line drug are proposed. It is essential to know their efficacy and security profiles, in order to decide which is the best option for each patient. AIM AND OBJECTIVES •To assess the reasons for changes in disease modifying drugs in MS patients in routine clinical practice. MATERIAL AND METHODS •We included patients with MS who changed their treatment between 23rd May 2018 and 26th March 2019. •The collected data were: Disease modifying drugs Reasons for treatment Duration of initial therapy before and after the modification modification RESULTS •42 patients had any treatment modification during the study period. •26 (62%) were women and mean age was 47 years (SD 9.3). •Median duration of previous treatment was 44 months (3-282). •Previous treatment was a first-line drug in 34 patients (81%) and modified treatment was a first-line drug in 24 (57%). -

Patient Focused Disease State and Assistance Programs
Patient Focused Disease State and Assistance Programs Medication Medication Toll-free Brand (Generic) Website number Additional Resources Allergy/Asthma Xolair (omalizumab) xolair.com 1-866-4-XOLAIR lung.org Cardiovascular Pradaxa (dabigatran) pradaxa.com 877-481-5332 heart.org Praluent (alirocumab) praluent.com 844-PRALUENT thefhfoundation.org Repatha (evolocumab) repatha.com 844-REPATHA Tikosyn (dofetilide) tikosyn.com 800-879-3477 Crohn’s Disease Cimzia (certolizumab pegol) cimzia.com 866-4-CIMZIA crohnsandcolitis.com Humira (adalimumab) humira.com 800-4-HUMIRA crohnsforum.com Stelara (ustekinumab) stelarainfo.com 877-STELARA Dermatology Cosentyx (secukinumab) cosentyx.com 844-COSENTYX psoriasis.org Dupixent (dupilumab) dupixent.com 844-DUPIXENT nationaleczema.org Enbrel (etanercept) enbrel.com 888-4-ENBREL Humira (adalimumab) humira.com 800-4-HUMIRA Otezla (apremilast) otezla.com 844-4-OTEZLA Stelara (ustekinumab) stelarainfo.com 877-STELARA Taltz (ixekizumab) taltz.com 800-545-5979 Hematology Aranesp (darbepoetin alfa) aranesp.com 805-447-1000 chemocare.com Granix (filgrastim) granixrx.com 888-4-TEVARX hematology.org Jadenu (deferasirox) jadenu.com 888-282-7630 Neulasta (pegfilgrastim) neulasta.com 800-77-AMGEN Neupogen (filgrastim) neupogen.com 800-77-AMGEN Nivestym (filgrastim) nivestym.com 800-879-3477 Zarxio (filgrastim) zarxio.com 800-525-8747 Zytiga (abiraterone) zytiga.com 800-JANSSEN Hepatitis B Baraclude (entecavir) baraclude.com 800-321-1335 cdc.gov Viread (tenofovir disoproxil viread.com 800-GILEAD-5 hepb.org fumarate) -

COMPARISON of the WHO ATC CLASSIFICATION & Ephmra/Intellus Worldwide ANATOMICAL CLASSIFICATION
COMPARISON OF THE WHO ATC CLASSIFICATION & EphMRA/Intellus Worldwide ANATOMICAL CLASSIFICATION: VERSION June 2019 2 Comparison of the WHO ATC Classification and EphMRA / Intellus Worldwide Anatomical Classification The following booklet is designed to improve the understanding of the two classification systems. The development of the two systems had previously taken place separately. EphMRA and WHO are now working together to ensure that there is a convergence of the 2 systems rather than a divergence. In order to better understand the two classification systems, we should pay attention to the way in which substances/products are classified. WHO mainly classifies substances according to the therapeutic or pharmaceutical aspects and in one class only (particular formulations or strengths can be given separate codes, e.g. clonidine in C02A as antihypertensive agent, N02C as anti-migraine product and S01E as ophthalmic product). EphMRA classifies products, mainly according to their indications and use. Therefore, it is possible to find the same compound in several classes, depending on the product, e.g., NAPROXEN tablets can be classified in M1A (antirheumatic), N2B (analgesic) and G2C if indicated for gynaecological conditions only. The purposes of classification are also different: The main purpose of the WHO classification is for international drug utilisation research and for adverse drug reaction monitoring. This classification is recommended by the WHO for use in international drug utilisation research. The EphMRA/Intellus Worldwide classification has a primary objective to satisfy the marketing needs of the pharmaceutical companies. Therefore, a direct comparison is sometimes difficult due to the different nature and purpose of the two systems. -

Updates in the Diagnosis and Treatment of Inflammatory Bowel Disease: Highlights from Digestive Disease Week 2011
August 2011 www.clinicaladvances.com Volume 7, Issue 8, Supplement 13 Updates in the Diagnosis and Treatment of Inflammatory Bowel Disease: Highlights From Digestive Disease Week 2011 A Review of Selected Presentations From Digestive Disease Week 2011 May 7–10, 2011 Chicago, Illinois With commentary by: Gary R. Lichtenstein, MD Director, Inflammatory Bowel Disease Program Professor of Medicine University of Pennsylvania Philadelphia, Pennsylvania A CME Activity Approved for 1.0 AMA PRA Category 1 Credit(s)TM Release date: August 2011 Expiration date: August 31, 2012 Estimated time to complete activity: 1.0 hour Supported through an educational grant from UCB, Inc. Sponsored by Postgraduate Institute for Medicine Target Audience: This activity has been designed to meet the Pharmaceuticals, Proctor & Gamble, Prometheus Laboratories, Inc., Salix educational needs of gastroenterologists who treat patients with Pharmaceuticals, Santarus, Schering-Plough Corporation, Shire Pharma- Crohn’s disease (CD) and/or ulcerative colitis (UC). ceuticals, UCB, Warner Chilcotte, and Wyeth. He has also received funds for contracted research from Alaven, Bristol-Myers Squibb, Centocor Ortho Statement of Need/Program Overview: Various abstracts were Biotech, Ferring, Proctor & Gamble, Prometheus Laboratories, Inc., Salix presented at Digestive Disease Week 2011. Unfortunately, physicians cannot Pharmaceuticals, Shire Pharmaceuticals, UCB, and Warner Chilcotte. attend all of the poster sessions in their therapeutic area, and some physicians may have been unable -

The Real-World Patient Experience of Fingolimod and Dimethyl Fumarate
Wicks et al. BMC Res Notes (2016) 9:434 DOI 10.1186/s13104-016-2243-8 BMC Research Notes RESEARCH ARTICLE Open Access The real‑world patient experience of fingolimod and dimethyl fumarate for multiple sclerosis Paul Wicks1* , Lawrence Rasouliyan2, Bo Katic1, Beenish Nafees3, Emuella Flood3 and Rahul Sasané4 Abstract Background: Oral disease-modifying therapies offer equivalent or superior efficacy and greater convenience versus injectable options. Objectives: To compare patient-reported experiences of fingolimod and dimethyl fumarate. Methods: Adult relapsing-remitting multiple sclerosis patients treated with fingolimod or dimethyl fumarate were recruited from an online patient community and completed an online survey about treatment side effects, discon- tinuation, and satisfaction. Results: 281 patients in four groups completed the survey: currently receiving fingolimod (CF, N 61), currently receiving dimethyl fumarate (CDMF, N 129), discontinued fingolimod (DF, N 32) and discontinued= dimethyl fuma- rate (DDMF, N 59). Reasons for treatment= switch were to take oral treatment =(CF: 63.3 %, CDMF: 61.8 %), side effects of prior medication= (CF: 67.3 %, CDMF: 44.1 %) and lack of effectiveness of prior medication (CF: 38.8 %, CDMF: 31.4 %). Main reasons for discontinuation were side effects (DF: 46.9 %, DDMF: 67.8 %) and lack of effectiveness (DF: 25.0 %, DDMF: 15.3 %). CDMF patients had an increased risk of abdominal pain, flushing, diarrhea, and nausea. Treatment satisfaction was highest among CF patients followed by CDMF, DF, and then DDMF patients. Conclusions: Discontinuation was driven by experience of side effects. Patients currently taking dimethyl fumarate were more likely to experience a side effect versus patients currently taking fingolimod. -

Fingolimod (Gilenya)
Fingolimod (Gilenya) This factsheet is about fingolimod, a Whether you’ll be offered this or any other DMT disease modifying therapy (DMT) for depends on whether you qualify for it based relapsing multiple sclerosis (MS). on guidelines used by your neurologist. These come from NICE and the Association of British At the end of this factsheet you’ll find out where Neurologists and are based on a drug’s Europe- you can get more information on this drug, other wide licence. drugs for MS and the benefits of early treatment. In England there are also rules from NHS England about who can have the different DMTs and when. This factsheet doesn’t cover everything about Scotland, Wales and Northern Ireland also have this drug and shouldn’t be used in place of their own guidelines for many DMTs. advice from your MS specialist team. For more information speak to them and read the online information from the drug’s makers (see the Whether you can have a drug also depends on section More information and support). if the NHS where you live will pay for it. NHS England guidelines on this tend to follow what What is fingolimod? NICE says. Fingolimod is a drug that was first given a licence These people can have fingolimod: to be used against relapsing MS in the UK in 2011. In England and Northern Ireland: In 2012 the National Institute for Health and Care Excellence (NICE) gave the go ahead for it to be • People with ‘highly active relapsing remitting used on the NHS. -

Delayed Allergic Reaction to Natalizumab Associated with Early Formation of Neutralizing Antibodies
OBSERVATION Delayed Allergic Reaction to Natalizumab Associated With Early Formation of Neutralizing Antibodies Markus Krumbholz, MD; Hannah Pellkofer, MD; Ralf Gold, MD; Lisa Ann Hoffmann, MD; Reinhard Hohlfeld, MD; Tania Ku¨mpfel, MD Background: Natalizumab is a new therapeutic option exanthema, and a swollen lower lip during several days for relapsing-remitting multiple sclerosis. As with other an- after his second infusion of natalizumab. tibody therapies, hypersensitivity reactions have been ob- served. In the Natalizumab Safety and Efficacy in Relapsing- Results: The patient developed a delayed, serum sick- RemittingMultipleSclerosis(AFFIRM)trial,infusion-related ness–like, type III systemic allergic reaction to natali- hypersensitivity reactions developed in 4% of patients, usu- zumab. Five weeks after initiation of this therapy, he tested ally within 2 hours after starting the infusion. positive for antinatalizumab antibodies and exhibited persistent antibody titers 8 and 12 weeks later. His symp- Objective: To report a significant, delayed, serum sick- toms completely resolved with a short course of oral ness–like, type III systemic allergic reaction to natali- glucocorticosteroids. zumab. Conclusion: Clinicians and patients should be alert not Design: Case report describing clinical follow-up and only to immediate but also to significantly delayed sub- the serial measurement of antinatalizumab antibodies. stantial allergic reactions to natalizumab. Patient: A 23-year-old man with relapsing-remitting mul- tiple sclerosis developed a fever, arthralgias, urticarial Arch Neurol. 2007;64(9):1331-1333 ATALIZUMAB IS A MONO- resulting in severe gait ataxia (Expanded clonal antibody targeting Disability Status Scale, 5.5). Previous treat- ␣ 4 integrin. It is highly ef- ments included steroid pulses for relapses fective in reducing the re- and 44 µg of interferon beta-1a 3 times lapse rate and disease pro- weekly. -

CIRCULAR 73 of 2015: Entry and Verification (E&V) Criteria for Identifying Beneficiaries with Risk Factors in Medical Scheme
CIRCULAR Reference: E&V version 9.1 Contact person: Carrie-Anne Cairncross Tel: 012 431 0412 Fax: 086 687 3979 E-mail: [email protected] Date: 11 December 2015 CIRCULAR 73 OF 2015: Entry and verification (E&V) criteria for identifying beneficiaries with risk factors in medical schemes The Council for Medical schemes (CMS) has published the guidelines for identifying medical scheme beneficiaries with risk factors in accordance with the entry and verification criteria. (http://www.medicalschemes.com/files/ITAP%20Documents/Vers9_1OfEVGdlns20151211.pdf) It is important that medical schemes and administrators take note of the updated version 9.1 and implement all the changes made in the guideline before they extract the data for the 2015 submission. The changes are listed below. 1. Parts 3 and 4, which relate to preparation and submission of data has been amended to reflect the submission of Scheme Risk Measurement (SRM) data via the Healthcare Utilisation Annual Statutory Returns (ASR) System. 2. Changes to clinical entry end verification criteria: 2.1. The provider type was changed from any registered medical practitioner to specialist ophthalmologist (Table 17) 2.2. Glaucoma was added to the list of conditions that need specialist diagnosis as stated in paragraph 5.17 2.3. Rheumatoid Arthritis was removed from paragraph 5.17 2.4. The note on the Rheumatoid Arthritis table i.e. “Where a patient is not using disease modifying anti-rheumatic medicines, the diagnosis must be verified by a specialist physician or rheumatologist” has been updated to state “Where a patient is using disease modifying anti-rheumatic medicines, the diagnosis must be verified by a specialist physician or rheumatologist.” 2.5.