Bjophthalmol-2020-316528 1..7
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

In Vivo Sublayer Analysis of Human Retinal Inner Plexiform Layer Obtained by Visible- Light Optical Coherence Tomography
bioRxiv preprint doi: https://doi.org/10.1101/2021.01.08.425925; this version posted January 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. In Vivo Sublayer Analysis Of Human Retinal Inner Plexiform Layer Obtained By Visible- Light Optical Coherence Tomography Zeinab Ghassabi*1, Roman V. Kuranov*2,3, Mengfei Wu1, Behnam Tayebi1,4, Yuanbo Wang3, Ian Rubinoff2, Xiaorong Liu5, Gadi Wollstein1,6, Joel S. Schuman1,6, Hao F. Zhang2, and Hiroshi Ishikawa1,6 1 Department of Ophthalmology, NYU Langone Health, New York, NY, United States. 2 Department of Biomedical Engineering, Northwestern University, Evanston, IL, United States. 3 Opticent Inc., Evanston, IL, United States. 4 Neuroscience Institute, NYU Langone Health, NY, United States. 5 Department of Biology, University of Virginia, Charlottesville, VA, United States 6 Department of Biomedical Engineering, New York University Tandon School of Engineering, New York, NY, United States Funding: NIH: R01-EY013178, R01EY029121, R01EY026078, R44EY026466 * These authors contributed equally to this work Correspondence author: Dr. Hiroshi Ishikawa, [email protected] bioRxiv preprint doi: https://doi.org/10.1101/2021.01.08.425925; this version posted January 10, 2021. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Purpose: Growing evidence suggests, in glaucoma, the dendritic degeneration of subpopulation of the retinal ganglion cells (RGCs) may precede RGCs soma death. Since different RGCs synapse in different IPL sublayers, visualization of the lamellar structure of the IPL could enable both clinical and fundamental advances in glaucoma understanding and management. -

Retinal Anatomy and Histology
1 Q Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? 2 Q/A Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. The term neurosensory retina refers to the neural lining on the inside of the eye, whereas the term retina refers to this neural lining along with the retinal pigmentthree epithelium words (RPE). 3 A Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. The term neurosensory retina refers to the neural lining on the inside of the eye, whereas the term retina refers to this neural lining along with the retinal pigment epithelium (RPE). 4 Q Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. The term neurosensory retina refers to the neural lining on the inside of the eye, whereas the term retina refers to this neural lining along with the retinal pigment epithelium (RPE). The neurosensory retina contains three classes of cells—what are they? There are five types of neural elements—what are they? What are the three types of glial cells? The two vascular cell types? --? ----PRs ----Bipolar cells ----Ganglion cells ----Amacrine cells ----Horizontal cells --? ----Müeller cells ----Astrocytes ----Microglia --? ----Endothelial cells ----Pericytes 5 A Retinal Anatomy and Histology What is the difference between the retina and the neurosensory retina? While often used interchangeably (including, on occasion, in this slide-set), these are technically not synonyms. -

Physiology of the Retina
PHYSIOLOGY OF THE RETINA András M. Komáromy Michigan State University [email protected] 12th Biannual William Magrane Basic Science Course in Veterinary and Comparative Ophthalmology PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS • NON-NEURONAL RETINAL CELLS • RETINAL BLOOD FLOW Retina ©Webvision Retina Retinal pigment epithelium (RPE) Photoreceptor segments Outer limiting membrane (OLM) Outer nuclear layer (ONL) Outer plexiform layer (OPL) Inner nuclear layer (INL) Inner plexiform layer (IPL) Ganglion cell layer Nerve fiber layer Inner limiting membrane (ILM) ©Webvision Inherited Retinal Degenerations • Retinitis pigmentosa (RP) – Approx. 1 in 3,500 people affected • Age-related macular degeneration (AMD) – 15 Mio people affected in U.S. www.nei.nih.gov Mutations Causing Retinal Disease http://www.sph.uth.tmc.edu/Retnet/ Retina Optical Coherence Tomography (OCT) Histology Monkey (Macaca fascicularis) fovea Ultrahigh-resolution OCT Drexler & Fujimoto 2008 9 Adaptive Optics Roorda & Williams 1999 6 Types of Retinal Neurons • Photoreceptor cells (rods, cones) • Horizontal cells • Bipolar cells • Amacrine cells • Interplexiform cells • Ganglion cells Signal Transmission 1st order SPECIES DIFFERENCES!! Photoreceptors Horizontal cells 2nd order Bipolar cells Amacrine cells 3rd order Retinal ganglion cells Visual Pathway lgn, lateral geniculate nucleus Changes in Membrane Potential Net positive charge out Net positive charge in PHYSIOLOGY OF THE RETINA • INTRODUCTION • PHOTORECEPTORS • OTHER RETINAL NEURONS -

Development of Synaptic Arrays in the Inner Plexiform Layer of Neonatal Mouse Retina
Development of Synaptic Arrays in the Inner Plexiform Layer of Neonatal Mouse Retina LESLIE J. FISHER The University of Michigan, Ann Arbor, Michigan 481 09 ABSTRACT Retinas from mice of the C57BL/6 strain were sampled at fre- quent intervals from birth to postnatal day 33 to determine the numerical den- sity of conventional and ribbon synapses within the inner plexiform layer (IPL) as a function of time. Synaptic arrays of the IPL were formed in three phases. During Phase I, from day 3 to day 10, conventional synapses were produced at a mean rate of 0.44 synapsesl1,OOO pm3/hour, but no ribbons were seen. During Phase 11, from day 11 to day 15, ribbons formed at a rate of 0.38 ribbons/1,000 pm3/hour and conventional synapses were produced at a rate of 1.15 synapsed 1,000 p.m31hour. Phase 111 began at day 15, the approximate time of eye open- ing in these animals, and was characterized by a sharp reduction in the rate of production of both ribbons and conventional synapses. During this phase rib- bons achieved a final mean density of 113 ribbons/1,000 prn' and conventionals achieved a final mean density of 250 synapses/1,000 fim3. Serial synapses ap- peared in Phase I1 but remained at low densities. The retinal inner plexiform layer (IPL) is MATERIALS AND METHODS particularly suitable for quantitative analysis A colony of the inbred strain of C57BL/6 because of its precisely defined borders, its mice originally purchased from Microbio- involvement with only three presynaptic neu- logical Associates was maintained under a ronal elements, and the ability to distinguish 14-hour light, 10-hour dark cycle with food ribbon from conventional synaptic complexes. -
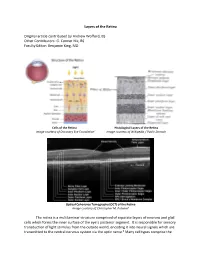
Layers of the Retina Original Article Contributed by Andrew Wofford, BS
Layers of the Retina Original article contributed by Andrew Wofford, BS Other Contributors: G. Conner Nix, BS Faculty Editor: Benjamin King, MD Cells of the Retina Histological Layers of the Retina Image courtesy of Discovery Eye Foundation1 Image courtesy of Wikipedia / Public Domain Optical Coherence Tomography (OCT) of the Retina Image courtesy of Christopher M. Putnam2 The retina is a multilaminar structure comprised of separate layers of neurons and glial cells which forms the inner surface of the eye’s posterior segment. It is responsible for sensory transduction of light stimulus from the outside world, encoding it into neural signals which are transmitted to the central nervous system via the optic nerve.3 Many cell types comprise the retina and allow it to perform its complex function; these include rods, cones, retinal ganglion cells, bipolar cells, Müller glial cells, horizontal cells, and amacrine cells.4 The ten layers of the retina from interior (bordering vitreous humor) to exterior (bordering choroid and sclera) are listed and described below.4,5 1. Inner Limiting Membrane – forms a barrier between the vitreous humor and the neurosensory retina. It is composed of the laterally branching foot plates of the Müller cells and is responsible for maintaining the structural integrity of the inner retina. • Clinical Correlation – The internal limiting membrane can be surgically removed for the treatment of several conditions, such as macular hole. 2. Nerve Fiber Layer – composed of ganglion cell axons that eventually converge to form the optic nerve. • Clinical Correlation – The nerve fiber layer and ganglion cell layers represent the part of the retina affected by glaucoma. -

Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical
Anatomy of the Globe 09 Hermann D. Schubert Basic and Clinical Science Course, AAO 2008-2009, Section 2, Chapter 2, pp 43-92. The globe is the home of the retina (part of the embryonic forebrain, i.e.neural ectoderm and neural crest) which it protects, nourishes, moves or holds in proper position. The retinal ganglion cells (second neurons of the visual pathway) have axons which form the optic nerve (a brain tract) and which connect to the lateral geniculate body of the brain (third neurons of the visual pathway with axons to cerebral cortex). The transparent media of the eye are: tear film, cornea, aqueous, lens, vitreous, internal limiting membrane and inner retina. Intraocular pressure is the pressure of the aqueous and vitreous compartment. The aqueous compartment is comprised of anterior(200ul) and posterior chamber(60ul). Aqueous and vitreous compartments communicate across the anterior cortical gel of the vitreous which seen from up front looks like a donut and is called the “annular diffusional gap.” The globe consists of two superimposed spheres, the corneal radius measuring 8mm and the scleral radius 12mm. The superimposition creates an external scleral sulcus, the outflow channels anterior to the scleral spur fill the internal scleral sulcus. Three layers or ocular coats are distinguished: the corneal scleral coat, the uvea and neural retina consisting of retina and pigmentedepithelium. The coats and components of the inner eye are held in place by intraocular pressure, scleral rigidity and mechanical attachments between the layers. The corneoscleral coat consists of cornea, sclera, lamina cribrosa and optic nerve sheath. -
Retinal Layers Handout
Retinal Layers Handout Vasculature Retinal Cells Retinal Layers Vascular Slabs Schematic Schematic Names and Abbrevations Names and Abbrevations Axon ILM Internal Limiting Membrane OCT Angiography Images Arterioles Vitreous Venules RNFL Cell Body Retinal Nerve Fiber Layer Müller Cell NFLVP GCL Ganglion Cell Layer SVC Dendrite Superficial Synaptic Vascular Terminal SVP Complex IPL Inner Plexiform Layer Axon Capillaries Capillaries Amacrine Cell ICP DVC Cell Body INL Inner Nuclear Layer Deep Inner Retina Dendrite Bipolar Cell Ganglion Cell Vascular NFLVP Capillaries Synaptic Complex Terminal OPL Outer Plexiform Layer DCP Horizontal Cell HFL+ Henle Fiber Layer + Retina Axon ONL Outer Nuclear Layer Müller Cell Cell Body ELM External Limiting Membrane AC Avascular SVP SVC Inner Photoreceptor Rod Complex Cone Segment EZ PR1 Layer of Inner Segment and Outer Retina Ellipsoid Zone Outer Segment Outer IZ PR2 Interdigitation Z. Segment Retinal Pigment Epithelium RPE RPE Bruch‘s Membrane BM Choriocapillaris Choriocapillaris BM CC Capillaries ICP Venules Arterioles Medium and Large Medium and Large CV Choroidal Choroidal Vessels Vessels Choroid Arteries Veins 210250-001 GL.AE20 © Heidelberg Engineering GmbH 210250-001 DCP DVC Fovea Optic Nerve Head www.he-academy.com Axon ILM Arterioles Internal Limiting Membrane Venules RNFL Cell Body Retinal Nerve Fiber Layer Müller Cell NFLVP GCL Ganglion Cell Layer SVC Dendrite Superficial Synaptic Vascular Terminal SVP Complex IPL Inner Plexiform Layer Axon Capillaries Capillaries Amacrine Cell ICP DVC Cell Body INL Inner Nuclear Layer Deep Inner Retina Dendrite Bipolar Cell Ganglion Cell Vascular Capillaries Synaptic Complex Terminal OPL Outer Plexiform Layer DCP Horizontal Cell HFL+ Henle Fiber Layer + Retina Axon ONL Outer Nuclear Layer Müller Cell Cell Body ELM External Limiting Membrane AC Avascular Inner Photoreceptor Rod Segment Complex Cone EZ PR1 Layer of Inner Segment and Outer Retina Ellipsoid Zone Outer Segment Outer IZ PR2 Interdigitation Z. -

Eye Structure and Chemical Details of the Retinal Layer of Juvenile Queen Danio Devario Regina (Fowler, 1934)
Kasetsart J. (Nat. Sci.) 49 : 711 - 716 (2015) Eye Structure and Chemical Details of the Retinal Layer of Juvenile Queen Danio Devario regina (Fowler, 1934) Piyakorn Boonyoung1, Sinlapachai Senarat2, Jes Kettratad2, Pisit Poolprasert3, Watiporn Yenchum4 and Wannee Jiraungkoorskul5,* ABSTRACT The eye structures and chemical details of the retinal layer in juvenile Queen Danio—Devario regina, an ornamental fish—were histologically investigated under a light microscope. Sample fish were collected from the Tapee River, Nakhon Si Thammarat province, Thailand and their heads were exclusively prepared using a standard histological technique. The results revealed that the eye of D. regina was composed of three layers—inner, middle and external—based on histological organization and cell types. The inner layer was composed of 10 layers; 1) pigment epithelium, 2) photoreceptor layer, 3) outer liming membrane, 4) outer nuclear layer, 5) outer plexiform layer, 6) inner nuclear layer, 7) inner plexiform layer, 8) ganglion cell layer, 9) optic nerve layer and 10) inner limiting membrane, respectively. The localization and chemical details showed that a periodic acid-Schiff reaction for the detection of glycoprotein was intensive in the pigment epithelial layer whereas the inner plexifrom layer had only a slight reaction. Reaction of aniline blue was employed for the detection of mucopolysaccharide which was slightly positive for three layers—the outer limiting membrane, outer plexiform and inner plexiform. Keywords: eye, histology, Devario regina, histochemistry INTRODUCTION cornea and the surrounding water and therefore, the lens has to do the majority of the refraction The eye is a specialized organ for the (Land and Nilsson, 2012). “Due to a refractive detection and analysis of light. -

Retinal Ganglion Cell and Inner Plexiform Layer Thickness Measurements AL De a Moura Et Al 1189
Eye (2012) 26, 1188–1193 & 2012 Macmillan Publishers Limited All rights reserved 0950-222X/12 www.nature.com/eye 1;2;3 1 1 CLINICAL STUDY Retinal ganglion cell AL de A Moura , AS Raza , MA Lazow , CG De Moraes4;5 and DC Hood1;6 and inner plexiform layer thickness measurements in regions of severe visual field sensitivity loss in 1Department of Psychology, patients with Columbia University, New York, NY, USA glaucoma 2Department of Experimental Psychology, University of Sa˜ o Paulo, Sa˜ o Paulo, Brazil Abstract Purpose To better understand the effects of RGC layer thickness exceeds the IP layer 3Department of Ophthalmology, ABC School severe glaucoma on the thickness of the retinal thickness in controls. of Medicine, Sa˜ o Paulo, ganglion cell (RGC) and inner plexiform (IP) Eye (2012) 26, 1188–1193; doi:10.1038/eye.2012.110; Brazil layers measured with frequency-domain optical published online 15 June 2012 coherence tomography. 4Einhorn Clinical Research Methods In experiment 1, macular cube Keywords: glaucoma; OCT; ganglion cell layer; Center, New York Eye and scans were obtained in 11 patients with inner plexiform layer Ear Infirmary, New York, NY, USA glaucoma and the thickness of both the RGC and IP layers were measured at locations Introduction 5Department of corresponding to 3, 5, and 71 eccentricity. For Ophthalmology, New York patients, only locations with total deviation Glaucomatous optic neuropathy has been University School of losses of À 15 dB or worse on perimetry were traditionally diagnosed based upon structural Medicine, New York, included. In experiment 2, higher resolution, (optic disc appearance) and functional (visual NY, USA horizontal midline scans were obtained from field (VF)) abnormalities. -

Nitric Oxide Synthase in Miiller Cells and Neurons of Salamander and Fish Retina
The Journal of Neuroscience, December 1994, 14(12): 7641-7654 Nitric Oxide Synthase in Miiller Cells and Neurons of Salamander and Fish Retina 6. A. Liepe,’ C. Stone,’ J. Koistinaho,’ and D. R. Copenhagen’ ‘Departments of Ophthalmology and Physiology, University of California, San Francisco, California 94143 and 2Department of Biochemistry & Biotechnology, University of Kuopio, FIN-7021 1 Kuopio, Finland Nitric oxide synthase (NOS) is the biosynthetic enzyme of molecule is synthesizedfrom I-arginine by nitric oxide synthase the signaling molecule nitric oxide (NO). NO donors have (NOS; Deguchi and Yoshioka, 1982; Palmer et al., 1988), and been reported to modulate conductances in cell types has been shown to activate soluble guanylyl cyclase (Arnold et throughout the retina, from photoreceptors to ganglion cells. al., 1977) and increaseADP-ribosylation of specificprotein sub- Previously, NOS immunoreactivity has been reported in ama- strates (Brtine and Lapetina, 1989; Zhang et al., 1994) in ap- crine cells and cells within the ganglion cell layer. Here, we propriate target cells. BecauseNO is a short-lived gaseousmol- have examined the cellular localization of NOS in the retinas ecule, it is generally assumedthat the synthetic enzyme NOS of salamander, goldfish, and catfish using both an affinity- must be located either in the target cells or in cells that are purified antiserum to brain NOS and NADPH diaphorase immediately adjacent to the target cells (for review, seeGarth- (NADPHd) histochemistry. These markers indicate that an Waite, 1991). Knowledge of the cell types containing NOS is an NOS-like enzyme is localized not only to presumptive ama- important factor in determining whether NO acts as a neuro- crine cells but also, depending on the species, to photore- modulator in viva The cellular localization of NOS has relied ceptor ellipsoids, to somata within the ganglion cell layer, heavily on both antisera directed against specific isoforms of and to horizontal cells. -

How the Retina Works
How the Retina Works Much of the construction of an image takes place in the retina itself through the use of specialized neural circuits Helga Kolb he retina is a filmy piece of tissue, fixed in the photoreceptors’ opsin pro- Most other mammalian retinas also Tbarely half a millimeter thick, that teins, where this small molecule have a preponderance of rods, and the lines the inside of the eyeball. The tis- changes its conformation in response cones are often concentrated in special- sue develops from a pouch of the em- to photons, or packets of light. Once ized regions. In species such as cats bryonic forebrain, and the retina is retinal molecules are exposed to light and dogs, images focus to a central therefore considered part of the brain. and undergo their conformational specialized area, aptly called the area This most important part of the eye change, they are recycled back into the centralis, where cones predominate. has a basic structure similar to that of a pigment epithelium. This tissue be- The retinas of mammals such as rab- three-layer cake, with the bodies of hind the retina is usually very dark bits and squirrels, as well as those of nerve cells arrayed in three rows sepa- because its cells are full of melanin nonmammals like turtles, have a long, rated by two layers packed with granules. The pigment granules ab- horizontal strip of specialized cells synaptic connections. The retina in- sorb stray photons, preventing their called a visual streak, which can detect cludes both the sensory neurons that reflection back into the photorecep- the fast movement of predators. -

Insomnia Might Influence the Thickness of Choroid, Retinal
brain sciences Article Insomnia Might Influence the Thickness of Choroid, Retinal Nerve Fiber and Inner Plexiform Layer Cigdem Sahbaz 1,* , Ahmet Elbay 2, Mine Ozcelik 3 and Hakan Ozdemir 2 1 Department of Psychiatry, Faculty of Medicine, Bezmialem Vakıf University, Istanbul 34093, Turkey 2 Department of Ophthalmology, Bezmialem Vakıf University, Istanbul 34093, Turkey; [email protected] (A.E.); [email protected] (H.O.) 3 School of Medicine, Bezmialem Vakıf University, Istanbul 34093, Turkey; [email protected] * Correspondence: [email protected] Received: 13 February 2020; Accepted: 17 March 2020; Published: 19 March 2020 Abstract: Sleep may play a fundamental role in retinal regulation and the degree of retinal variables. However, no clinical study has investigated optical coherence tomography (OCT) parameters in patients with primary insomnia. All participants were evaluated with the insomnia severity index (ISI) and the Pittsburgh sleep quality index (PSQI). The retinal nerve fiber layer (RNFL), ganglion cell layer (GC), inner plexiform layer (IPL), macula and choroidal (CH) thickness were compared between 52 drug-naïve patients with primary insomnia and 45 age-gender-BMI-smoke status matched healthy controls (HC). The patients with primary insomnia differed from the HC regarding RNFL-Global (p = 0.024) and RNFL-Nasal inferior (p = 0.010); IPL-Temporal (p < 0.001), IPL-Nasal (p < 0.001); CH-Global (p < 0.001), CH-Temporal (p = 0.004), CH-Nasal (p < 0.001), and CH-Fovea (p = 0.019). ISI correlated with RNFL-Global and RNFL-Nasal inferior. The regression analysis revealed that ISI was the significant predictor for the thickness of RNFL- Nasal inferior (p = 0.020), RNFL-Global (p = 0.031), and CH-Nasal (p = 0.035) in patients with primary insomnia.