Sperm Features of Captive Atlantic Bluefin Tuna (Thunnus Thynnus)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Aspects of the Life History of Hornyhead Turbot, Pleuronichthys Verticalis, Off Southern California
Aspects of the Life History of Hornyhead Turbot, Pleuronichthys verticalis, off Southern California he hornyhead turbot T(Pleuronichthys verticalis) is a common resident flatfish on the mainland shelf from Magdalena Bay, Baja Califor- nia, Mexico to Point Reyes, California (Miller and Lea 1972). They are randomly distributed over the bottom at a density of about one fish per 130 m2 and lie partially buried in the sediment (Luckinbill 1969). Hornyhead turbot feed primarily on sedentary, tube-dwelling polychaetes (Luckinbill 1969, Allen 1982, Cross et al. 1985). They pull the tubes from the sediment, Histological section of a fish ovary. extract the polychaete, and then eject the tube (Luckinbill 1969). Hornyhead turbot are Orange County, p,p’-DDE Despite the importance of batch spawners and may averaged 362 μg/kg wet the hornyhead turbot in local spawn year round (Goldberg weight in hornyhead turbot monitoring programs, its life 1982). Their planktonic eggs liver and 5 μg/kg dry weight in history has received little are 1.00-1.16 mm diameter the sediments (CSDOC 1992). attention. The long-term goal (Sumida et al. 1979). Their In the same year in Santa of our work is to determine larvae occur in the nearshore Monica Bay, p,p’-DDE aver- how a relatively low trophic plankton throughout the year aged 7.8 mg/kg wet weight in level fish like the hornyhead (Gruber et al. 1982, Barnett et liver and 81 μg/kg dry weight turbot accumulates tissue al. 1984, Moser et al. 1993). in the sediments (City of Los levels of chlorinated hydrocar- Several agencies in South- Angeles 1992). -

Snakeheadsnepal Pakistan − (Pisces,India Channidae) PACIFIC OCEAN a Biologicalmyanmar Synopsis Vietnam
Mongolia North Korea Afghan- China South Japan istan Korea Iran SnakeheadsNepal Pakistan − (Pisces,India Channidae) PACIFIC OCEAN A BiologicalMyanmar Synopsis Vietnam and Risk Assessment Philippines Thailand Malaysia INDIAN OCEAN Indonesia Indonesia U.S. Department of the Interior U.S. Geological Survey Circular 1251 SNAKEHEADS (Pisces, Channidae)— A Biological Synopsis and Risk Assessment By Walter R. Courtenay, Jr., and James D. Williams U.S. Geological Survey Circular 1251 U.S. DEPARTMENT OF THE INTERIOR GALE A. NORTON, Secretary U.S. GEOLOGICAL SURVEY CHARLES G. GROAT, Director Use of trade, product, or firm names in this publication is for descriptive purposes only and does not imply endorsement by the U.S. Geological Survey. Copyrighted material reprinted with permission. 2004 For additional information write to: Walter R. Courtenay, Jr. Florida Integrated Science Center U.S. Geological Survey 7920 N.W. 71st Street Gainesville, Florida 32653 For additional copies please contact: U.S. Geological Survey Branch of Information Services Box 25286 Denver, Colorado 80225-0286 Telephone: 1-888-ASK-USGS World Wide Web: http://www.usgs.gov Library of Congress Cataloging-in-Publication Data Walter R. Courtenay, Jr., and James D. Williams Snakeheads (Pisces, Channidae)—A Biological Synopsis and Risk Assessment / by Walter R. Courtenay, Jr., and James D. Williams p. cm. — (U.S. Geological Survey circular ; 1251) Includes bibliographical references. ISBN.0-607-93720 (alk. paper) 1. Snakeheads — Pisces, Channidae— Invasive Species 2. Biological Synopsis and Risk Assessment. Title. II. Series. QL653.N8D64 2004 597.8’09768’89—dc22 CONTENTS Abstract . 1 Introduction . 2 Literature Review and Background Information . 4 Taxonomy and Synonymy . -

Food Choice of Different Size Classes of Flounder (Platichthys Flesus ) In
Food choice of different size classes of flounder ( Platichthys flesus ) in the Baltic Sea Jennie Ljungberg Degree project in biology, Master of science (2 years), 2014 Examensarbete i biologi 30 hp till masterexamen, 2014 Biology Education Centre Supervisor: Bertil Widbom Table of Contents ABSTRACT ............................................................................................................................................ 3 INTRODUCTION ................................................................................................................................... 4 Flounders in the Baltic Sea .................................................................................................................. 5 The diet of flounders ........................................................................................................................... 6 Blue mussel (Mytilus edulis) ............................................................................................................... 7 Blue mussels in the Baltic Sea............................................................................................................. 8 The nutritive value of blue mussels ..................................................................................................... 9 The condition of flounders in the Baltic Sea ....................................................................................... 9 Aims ................................................................................................................................................. -

Greenland Turbot Assessment
6HFWLRQ STOCK ASSESSMENT OF GREENLAND TURBOT James N. Ianelli, Thomas K. Wilderbuer, and Terrance M. Sample 6XPPDU\ Changes to this year’s assessment in the past year include: 1. new summary estimates of retained and discarded Greenland turbot by different target fisheries, 2. update the estimated catch levels by gear type in recent years, and 3. new length frequency and biomass data from the 1998 NMFS eastern Bering Sea shelf survey. Conditions do not appear to have changed substantively over the past several years. For example, the abundance of Greenland turbot from the eastern Bering Sea (EBS) shelf-trawl survey has found only spotty quantities with very few small fish that were common in the late 1970s and early 1980s. The majority of the catch has shifted to longline gear in recent years. The assessment model analysis was similar to last year but with a slightly higher estimated overall abundance. We attribute this to a slightly improved fit to the longline survey data trend. The target stock size (B40%, female spawning biomass) is estimated at about 139,000 tons while the projected 1999 spawning biomass is about 110,000 tons. The adjusted yield projection from F40% computations is estimated at 20,000 tons for 1999, and increase of 5,000 from last year’s ABC. Given the continued downward abundance trend and no sign of recruitment to the EBS shelf, extra caution is warranted. We therefore recommend that the ABC be set to 15,000 tons (same value as last year). As additional survey information become available and signs of recruitment (perhaps from areas other than the shelf) are apparent, then we believe that the full ABC or increases in harvest may be appropriate for this species. -

Plaice (Pleuronectes Platessä) Contents
1-group plaice (Pleuronectes platessä) Contents Acknowledgements:............................................................................................................ 1 Abstract:.............................................................................................................................3 Chapter 1: General introduction.....................................................................................................4 Chapter 2: Fin-ray count variation in 0-group flatfish: plaice (Pleuronectesplatessa (L.)) and flounder (Platichthys flesus ( L.)) on the west coast of Ireland..............................15 Chapter 3: Variation in the fin ray counts of 0-group turbot (Psetta maxima L.) and brill (Scophthalmus rhombus L.) on the west coast of Ireland: 2006-2009.......................... 28 Chapter 4: Annual and spatial variation in the abundance length and condition of turbot (.Psetta maxima L.) on nursery grounds on the west coast of Ireland: 2000-2007.........41 Chapter 5: Variability in the early life stages of juvenile plaice (.Pleuronectes platessa L.) on west of Ireland nursery grounds; 2000 - 2007........................................................64 Chapter 6: The early life history of turbot (Psetta maxima L.) on nursery grounds along the west coast of Ireland: 2007 -2009, as described by otolith microstructure.............85 Chapter 7: The feeding ecology of 0-group turbot (Psetta maxima L.) and brill (Scophthalmus rhombus L.) on Irish west coast nursery grounds.................................96 Chapter -

Failing Fish
Failing Fish ----Advertisement---- ----Advertisement---- HOME Failing Fish NEWS COMMENTARY News: A sampling of creatures at serious risk of disappearing from our oceans and our dinner plates ARTS MOJOBLOG Illustrations by Jack Unruh RADIO CUSTOMER March/April 2006 Issue SERVICE DONATE STORE ABOUT US NEWSLETTERS SUBSCRIBE ADVERTISE Bluefin Tuna Warm-blooded bluefins, which can weigh 1,500 punds, are one of the largest bony fish swimming the seas. The Atlantic bluefin population has fallen by more than 80 percent since the 1970s; Pacific stocks are also dwindling. Advanced Search Browse Back Issues http://www.motherjones.com/news/feature/2006/03/failing_fish.html (1 of 4)2/23/2006 1:30:09 PM Failing Fish Read the Current Issue BUY THIS ISSUE SUBSCRIBE NOW Blue Crab Since Chesapeake Bay harvests are half of what they were a decade ago, at least 70 percent of crabmeat CRAZY PRICE! products sold in the United States now contain foreign crabs. 1 year just $10 Click Here Sundays on Air America Radio THIS WEEK The roots of the Eastern Oyster conflict over the Ships in the Chesapeake Bay once had to steer around massive oyster reefs. Poor water quality, exotic Danish Mohammed parasites, and habitat destruction have reduced the Chesapeake oyster stock to 1 percent of its historic level. cartoons, Clinton's economic advisor on Bush's troubles, and Iraq war veterans running for office as Democrats..... Learn More... Blue Marlin Since longlines replaced harpoons in the early 1960s, the Atlantic blue marlin has been driven toward extinction. A quarter of all blue marlin snared by longlines are dead by the time they reach the boat. -

Estimation of Age Profiles of Southern Bluefin Tuna
ESTIMATION OF AGE PROFILES OF SOUTHERN BLUEFIN TUNA Richard Morton* Mark Bravington* *CSIRO Maths and Information Science CCSBT-ESC/0309/32 Estimation of age profiles of Southern Bluefin Tuna Table of Contents ABSTRACT ..................................................................................................................1 1 Introduction ...........................................................................................................1 2 A likelihood framework for length and age-at-length data.....................................2 2.1 Estimating proportions-at-age ........................................................................4 2.1.1 Age-length key......................................................................................4 2.1.2 Iterated age-length key .........................................................................5 2.1.3 Parametric estimator: known growth.....................................................5 2.1.4 Parametric estimator: unknown growth.................................................6 2.2 Example: application to Greenland turbot data ..............................................6 3 Application to sampling design for sbt...................................................................7 3.1 Results by method .........................................................................................8 3.2 Results by fishery...........................................................................................9 3.2.2 Effect of varying the subsampling pattern...........................................10 -

Guide to Seafood
Bay Aquarium Bay PCF ( processed chlorine free) paper free) chlorine processed ( PCF Printed on 100% PCW (post consumer waste) and and waste) consumer (post PCW 100% on Printed Created in collaboration with the Monterey Monterey the with collaboration in Created www.seachoice.org choose another Best Choice item. item. Choice Best another choose codes on the guide. If you’re not sure, sure, not you’re If guide. the on codes Then, check the listings and colour colour and listings the check Then, • How was it farmed or caught? caught? or farmed it was How • • Where is this seafood from? seafood this is Where • • Is it farmed or wild? or farmed it Is • seafood smarts. seafood Guide Guide and don’t forget to share your your share to forget don’t and • What species is this? is species What • www.seachoice.org , , at assessments shop or dine: or shop seafood items, updates, and full full and updates, items, seafood and always ask questions when you you when questions ask always and Seafood Seafood But don’t stop here! Find more more Find here! stop don’t But bolded terms). Be sure to read labels labels read to sure Be terms). bolded it was caught or farmed (look for the the for (look farmed or caught was it oceans and communities healthy. healthy. communities and oceans one column based on how and where where and how on based column one Canadian Parks and Wilderness Society Wilderness and Parks Canadian Canada’s Canada’s Canada’s Canada’s our keep help and restaurant, or Some items are listed in more than than more in listed are items Some consumer power at the grocery store store grocery the at power consumer items in the lighter section below. -

Pictorial Guide to the Gill Arches of Gadids and Pleuronectids in The
Alaska Fisheries Science Center National Marine Fisheries Service U.S. DEPARTMENT OF COMMERCE AFSC PROCESSED REPORT 91.15 Pictorial Guide to the G¡ll Arches of Gadids and Pleuronectids in the Eastern Bering Sea May 1991 This report does not const¡Ute a publicalion and is for lnformation only. All data herein are to be considered provisional. ERRATA NOTICE This document is being made available in .PDF format for the convenience of users; however, the accuracy and correctness of the document can only be certified as was presented in the original hard copy format. Inaccuracies in the OCR scanning process may influence text searches of the .PDF file. Light or faded ink in the original document may also affect the quality of the scanned document. Pictorial Guide to the ciII Arches of Gadids and Pleuronectids in the Eastern Beri-ng Sea Mei-Sun Yang Alaska Fisheries Science Center National Marine Fisheries Se:nrice, NoAÀ 7600 Sand Point Way NE, BIN C15700 Seattle, lÍA 98115-0070 May 1991 11I ABSTRÀCT The strrrctures of the gill arches of three gadids and ten pleuronectids were studied. The purPose of this study is, by using the picture of the gill arches and the pattern of the gi[- rakers, to help the identification of the gadids and pleuronectids found Ín the stomachs of marine fishes in the eastern Bering Sea. INTRODUCTION One purjose of the Fish Food Habits Prograrn of the Resource Ecology and FisherY Managenent Division (REF
Management of Shark Fin Trade to and from Australia
MANAGEMENT Scalloped hammerhead sharks OF SHARK FIN TRADE TO AND FROM AUSTRALIA In preparing this report the author has made all reasonable efforts to ensure the information it contains is based on evidence. The TABLE OF CONTENTS views expressed in this report are those of the author based on that evidence. The author does not guarantee that there is not further evidence relevant to the matters covered 1. Executive Summary 2 by this report and therefore urges those with an interest in these matters to conduct their own due 2. Introduction 5 diligence and to draw their own conclusions. 3. Shark Fin Trade 7 Trends in shark fin trade 8 Australian shark fin trade 13 4. Shark Fishery Management 17 5. Fins Naturally Attached 23 6. Traceability 29 Principle 1: Unique Identification 31 Principle 2: Data Capture and Management 31 Principle 3: Data Communication 33 7. Managing International Trade 34 8. Conclusion 36 Annex A – Protected Species as of October 2020 38 Annex B – Country Specific HS Codes for Shark Fin 40 Annex C – Fisheries Specific Shark Management Measures 45 EXECUTIVE SUMMARY Healthy shark populations are an indicator of the health of the marine environment. Sharks play This report looks at the current trends in the global a key role in marine and estuarine environments, and people around the world rely on healthy shark fin trade and actions that Australia can take marine ecosystems for their livelihoods. It has been predicted that by 2033, shark based eco- to drive improvement in the shark fin industry, tourism will be worth more than 785 million USD. -

Sperm Cryopreservation
animals Article Effects of Cryoprotective Medium Composition, Dilution Ratio, and Freezing Rates on Spotted Halibut (Verasper variegatus) Sperm Cryopreservation Irfan Zidni 1 , Yun Ho Lee 1, Jung Yeol Park 1, Hyo Bin Lee 1, Jun Wook Hur 2 and Han Kyu Lim 1,* 1 Department of Marine and Fisheries Resources, Mokpo National University, Mokpo 58554, Korea; [email protected] (I.Z.); [email protected] (Y.H.L.); [email protected] (J.Y.P.); [email protected] (H.B.L.) 2 Faculty of Marine Applied Biosciences, Kunsan National University, 558 Daehak-ro, Gunsan, Jeonbuk 54150, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-61-450-2395; Fax: +82-61-452-8875 Received: 12 October 2020; Accepted: 16 November 2020; Published: 19 November 2020 Simple Summary: The spotted halibut, Verasper variegatus, is a popular fish species occurring naturally in the East China Sea and coastal areas of Korea and Japan. However, when reared in captivity, male and female spotted halibut do not usually mature synchronously. Maintaining production of this commercial fish in hatcheries through sperm cryopreservation is important. This study investigated the effect of several factors for successful cryopreservation of fish sperm including cryoprotective agents (CPAs), diluents, dilution ratios (Milt: CPA + diluents), and freezing rates. The observed factors significantly affected movable sperm ratio (MSR), sperm activity index (SAI), survival rate, and DNA damage after cryopreservation. In the present study, the mixture of 15% dimethyl sulfoxide (DMSO) + 300 mM sucrose with a dilution ratio lower than 1:2 and a freezing rate slower than 5 C/min provided the best treatment and reduced DNA damage. -
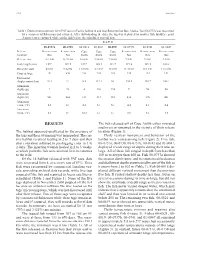
Pop-Up Archival Transmitting (PAT) Tags: a Method to Investigate Eh
128 Articles Table 1. Deployment summary for 8 PAT tags on Pacifi c halibut in and near Resurrection Bay, Alaska. Tag 00-0737a was recovered by a commercial fi sherman and returned. After downloading the data, the tag was deployed on another fi sh. Boldface print denotes tags recaptured while on the fi sh before the scheduled pop-off date. PAT # 00-0737a 00-0738 00-0818 00-0819 00-0821 00-0737b 00-0741 01-0047 Release Resurrection Resurrection Cape Cape Cape Resurrection Resurrection Resurrection location Bay Bay Aialik Aialik Aialik Bay Bay Bay Release date 11/21/00 11/21/00 3/16/01 3/16/01 3/16/01 7/5/01 7/5/01 7/5/01 Fish length (cm) 129.5 129.5 129.5 165.1 121.9 119.4 109.2 108.0 Recovery date 4/5/01 9/22/02 11/15/01 11/15/01 11/5/01 11/15/01 11/15/01 11/15/01 Days at large 135 670 244 244 234 133 133 133 Horizontal displacement (km) 20.3 2.5 6.5 112.1 0.0 336.9 190.7 358.3 Minimum depth (m) 2 26 4 136 174 72 96 80 Maximum depth (m) 502 466 212 212 210 436 396 404 Minimum temp. (°C) 4.3 4.5 4.8 5.4 5.4 4.4 4.8 4.8 Maximum temp. (°C) 8.6 8.3 12.2 6.4 6.3 8.8 7.4 7.8 RESULTS The fi sh released off of Cape Aialik either migrated southwest or remained in the vicinity of their release The halibut appeared unaffected by the presence of location (Figure 1).