Complex Molecules in W51 North Region
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Gas Phase Formation of C-Sic3 Molecules in the Circumstellar Envelope of Carbon Stars
Gas phase formation of c-SiC3 molecules in the circumstellar envelope of carbon stars Tao Yanga,b,1,2, Luke Bertelsc,1, Beni B. Dangib,3, Xiaohu Lid,e, Martin Head-Gordonc,2, and Ralf I. Kaiserb,2 aState Key Laboratory of Precision Spectroscopy, East China Normal University, Shanghai 200062, P. R. China; bDepartment of Chemistry, University of Hawai‘iatManoa, Honolulu, HI 96822; cDepartment of Chemistry, University of California, Berkeley, CA 94720; dXinjiang Astronomical Observatory, Chinese Academy of Sciences, Urumqi, Xinjiang 830011, P. R. China; and eNational Astronomical Observatories, Chinese Academy of Sciences, Beijing 100012, P. R. China Contributed by Martin Head-Gordon, May 17, 2019 (sent for review July 20, 2018; reviewed by Piergiorgio Casavecchia and David Clary) Complex organosilicon molecules are ubiquitous in the circumstel- Modern astrochemical models propose that the very first lar envelope of the asymptotic giant branch (AGB) star IRC+10216, silicon–carbon bonds are formed in the inner envelope of the but their formation mechanisms have remained largely elusive until carbon star, which is undergoing mass loss at the rates of several − now. These processes are of fundamental importance in initiating a 10 5 solar masses per year (22, 23). Pulsations from the central chain of chemical reactions leading eventually to the formation of star may initiate shocks, which (photo)fragment the circumstellar — organosilicon molecules among them key precursors to silicon car- materials (24, 25). These nonequilibrium conditions cause tem- — bide grains in the circumstellar shell contributing critically to the peratures of 3,500 K or above (19) and lead to highly reactive galactic carbon and silicon budgets with up to 80% of the ejected metastable fragments such as electronically excited silicon atoms, materials infused into the interstellar medium. -

Rotational Spectroscopy of the Isotopic Species of Silicon Monosulfide, Sisw
PAPER www.rsc.org/pccp | Physical Chemistry Chemical Physics Rotational spectroscopy of the isotopic species of silicon monosulfide, SiSw H. S. P. Mu¨ller,*ab M. C. McCarthy,cd L. Bizzocchi,e H. Gupta,cdf S. Esser,g H. Lichau,a M. Caris,a F. Lewen,a J. Hahn,g C. Degli Esposti,e S. Schlemmera and P. Thaddeuscd Received 2nd January 2007, Accepted 23rd January 2007 First published as an Advance Article on the web 20th February 2007 DOI: 10.1039/b618799d Pure rotational transitions of silicon monosulfide (28Si32S) and its rare isotopic species have been observed in their ground as well as vibrationally excited states by employing Fourier transform microwave (FTMW) spectroscopy of a supersonic molecular beam at centimetre wavelengths (13–37 GHz) and by using long-path absorption spectroscopy at millimetre and submillimetre wavelengths (127–925 GHz). The latter measurements include 91 transition frequencies for 28Si32S, 28Si33S, 28Si34S, 29Si32S and 30Si32Sinu = 0, as well as 5 lines for 28Si32Sinu = 1, with rotational quantum numbers J00 r 52. The centimetre-wave measurements include more than 300 newly recorded lines. Together with previous data they result in almost 600 transitions (J00 =0 and 1) from all twelve possible isotopic species, including 29Si36S and 30Si36S, which have fractional abundances of about 7 Â 10À6 and 4.5 Â 10À6, respectively. Rotational transitions were observed from u = 0 for the least abundant isotopic species to as high as u = 51 for the main species. Owing to the high spectral resolution of the FTMW spectrometer, hyperfine structure from the nuclear electric quadrupole moment of 33S was resolved for species containing this isotope, as was much smaller nuclear spin-rotation splitting for isotopic species involving 29Si. -

Standard Thermodynamic Properties of Chemical
STANDARD THERMODYNAMIC PROPERTIES OF CHEMICAL SUBSTANCES ∆ ° –1 ∆ ° –1 ° –1 –1 –1 –1 Molecular fH /kJ mol fG /kJ mol S /J mol K Cp/J mol K formula Name Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Crys. Liq. Gas Ac Actinium 0.0 406.0 366.0 56.5 188.1 27.2 20.8 Ag Silver 0.0 284.9 246.0 42.6 173.0 25.4 20.8 AgBr Silver(I) bromide -100.4 -96.9 107.1 52.4 AgBrO3 Silver(I) bromate -10.5 71.3 151.9 AgCl Silver(I) chloride -127.0 -109.8 96.3 50.8 AgClO3 Silver(I) chlorate -30.3 64.5 142.0 AgClO4 Silver(I) perchlorate -31.1 AgF Silver(I) fluoride -204.6 AgF2 Silver(II) fluoride -360.0 AgI Silver(I) iodide -61.8 -66.2 115.5 56.8 AgIO3 Silver(I) iodate -171.1 -93.7 149.4 102.9 AgNO3 Silver(I) nitrate -124.4 -33.4 140.9 93.1 Ag2 Disilver 410.0 358.8 257.1 37.0 Ag2CrO4 Silver(I) chromate -731.7 -641.8 217.6 142.3 Ag2O Silver(I) oxide -31.1 -11.2 121.3 65.9 Ag2O2 Silver(II) oxide -24.3 27.6 117.0 88.0 Ag2O3 Silver(III) oxide 33.9 121.4 100.0 Ag2O4S Silver(I) sulfate -715.9 -618.4 200.4 131.4 Ag2S Silver(I) sulfide (argentite) -32.6 -40.7 144.0 76.5 Al Aluminum 0.0 330.0 289.4 28.3 164.6 24.4 21.4 AlB3H12 Aluminum borohydride -16.3 13.0 145.0 147.0 289.1 379.2 194.6 AlBr Aluminum monobromide -4.0 -42.0 239.5 35.6 AlBr3 Aluminum tribromide -527.2 -425.1 180.2 100.6 AlCl Aluminum monochloride -47.7 -74.1 228.1 35.0 AlCl2 Aluminum dichloride -331.0 AlCl3 Aluminum trichloride -704.2 -583.2 -628.8 109.3 91.1 AlF Aluminum monofluoride -258.2 -283.7 215.0 31.9 AlF3 Aluminum trifluoride -1510.4 -1204.6 -1431.1 -1188.2 66.5 277.1 75.1 62.6 AlF4Na Sodium tetrafluoroaluminate -

ARTICLE Designing Isoelectronic Counterparts to Layered Group V Semiconductors Zhen Zhu, Jie Guan, Dan Liu, and David Toma´Nek*
ARTICLE Designing Isoelectronic Counterparts to Layered Group V Semiconductors Zhen Zhu, Jie Guan, Dan Liu, and David Toma´nek* Physics and Astronomy Department, Michigan State University, East Lansing, Michigan 48824, United States ABSTRACT In analogy to IIIÀV compounds, which have significantly broadened the scope of group IV semiconductors, we propose a class of IVÀVI compounds as isoelectronic counterparts to layered group V semiconductors. Using ab initio density functional theory, we study yet unrealized structural phases of silicon monosulfide (SiS). We find the black-phosphorus-like R-SiS to be almost equally stable as the blue-phosphorus-like β-SiS. Both R-SiS and β- SiS monolayers display a significant, indirect band gap that depends sensitively on the in-layer strain. Unlike 2D semiconductors of group V elements with the corresponding nonplanar structure, different SiS allotropes show a strong polarization either within or normal to the layers. We find that SiS may form both lateral and vertical heterostructures with phosphorene at a very small energy penalty, offering an unprecedented tunability in structural and electronic properties of SiS-P compounds. KEYWORDS: silicon sulfide . isoelectronic . phosphorene . ab initio . electronic band structure wo-dimensional semiconductors of monosulfide (SiS). We use ab initio density Tgroup V elements, including phos- functional theory (DFT) to identify stable phorene and arsenene, have been allotropes and determine their equilibrium rapidly attracting interest due to their sig- geometry and electronic structure. We have nificant fundamental band gap, large den- identified two nearly equally stable allo- sity of states near the Fermi level, and high tropes, namely, the black-phosphorus-like and anisotropic carrier mobility.1À4 Combi- R-SiS and the blue-phosphorus-like β-SiS, nation of these properties places these and show their structure in Figure 1a and d. -

Inorganic Seminar Abstracts
C 1 « « « • .... * . i - : \ ! -M. • ~ . • ' •» »» IB .< L I B RA FLY OF THE. UN IVERSITY Of 1LLI NOIS 546 1^52-53 Return this book on or before the Latest Date stamped below. University of Illinois Library «r L161— H41 Digitized by the Internet Archive in 2012 with funding from University of Illinois Urbana-Champaign http://archive.org/details/inorganicsemi195253univ INORGANIC SEMINARS 1952 - 1953 TABLE OF CONTENTS 1952 - 1953 Page COMPOUNDS CONTAINING THE SILICON-SULFUR LINKAGE 1 Stanley Kirschner ANALYTICAL PROCEDURES USING ACETIC ACID AS A SOLVENT 5 Donald H . Wilkins THE SOLVENT PHOSPHORYL CHLORIDE, POCl 3 12 S.J. Gill METHODS FOR PREPARATION OF PURE SILICON 17 Alex Beresniewicz IMIDODISULFINAMIDE 21 G.R. Johnston FORCE CONSTANTS IN POLYATOMIC MOLECILES 28 Donn D. Darsow METATHESIS IN LIQUID ARSENIC TRICHLORIDE 32 Harold H. Matsuguma THE RHENI DE OXIDATION STATE 40 Robert L. Rebertus HALOGEN CATIONS 45 L.H. Diamond REACTIONS OF THE NITROSYL ION 50 M.K. Snyder THE OCCURRENCE OF MAXIMUM OXIDATION STATES AMONG THE FLUOROCOMPLEXES OF THE FIRST TRANSITION SERIES 56 D.H. Busch POLY- and METAPHOSPHATES 62 V.D. Aftandilian PRODUCTION OF SILICON CHLORIDES BY ELECTRICAL DISCHARGE AND HIGH TEMPERATURE TECHNIQUES 67 VI. £, Cooley FLUORINE CONTAINING OXYHALIDES OF SULFUR 72 E.H. Grahn PREPARATION AND PROPERTIES OF URANYL CARBONATES 76 Richard *• Rowe THE NATURE OF IODINE SOLUTIONS 80 Ervin c olton SOME REACTIONS OF OZONE 84 Barbara H. Weil ' HYDRAZINE BY ELECTROLYSIS IN LIQUID AMMONIA 89 Robert N. Hammer NAPHTHAZARIN COMPLEXES OF THORIUM AND RARE EARTH METAL IONS 93 Melvin Tecotzky THESIS REPORT 97 Perry Kippur ION-PAIR FORMATION IN ACETIC ACID 101 M.M. -

United States Patent (19) 11 Patent Number: 5,843,391 Yamamoto Et Al
USOO584,3391A United States Patent (19) 11 Patent Number: 5,843,391 Yamamoto et al. (45) Date of Patent: Dec. 1, 1998 54 METHOD OF MANUFACTURING SILICON Mellor, “A Comprehensive Treatise on Inorganic and Theo SULFIDE retical Chemistry” vol., VI, QD31 M4, 1947 (no month), pp. 985-990. 75 Inventors: Kazutomi Yamamoto; Nobuhiko Ikeda, both of Hino, Japan European Search Report dated Jun. 23, 1997 for European 73 Assignee: Furukawa Co., Ltd., Tokyo, Japan Applin. No. EP97105989. Chemiker Zeitung, vol. 107, No. 10; 1983 Heidelberg DE, 21 Appl. No.: 839,677 pp. 289-290, R.G. Sobott et al. (no month). 22 Filed: Apr. 15, 1997 “Gmelins Handbuch De Anorganischer Chemie 8" Com 30 Foreign Application Priority Data pletely reworked edition Silicium Part B System-No. 15” 1959 Verlay Chemie, GmbH, p. 747, line 5-line 8. (no Apr. 16, 1996 JP Japan .................................... 8-094396 month). 51) Int. Cl. ............................ C01B33/00; CO1B 17/00 52 U.S. Cl. .......................... 423/344; 423/324; 423/565; 423/DIG. 12 Primary Examiner Ngoc-Yen Nguyen 58 Field of Search ..................................... 423/565,344, Attorney, Agent, or Firm Young & Basile, P.C. 423/324, DIG. 12 57 ABSTRACT 56) References Cited Silicon sulfide is manufactured from the fine powder of U.S. PATENT DOCUMENTS Silicon having a particle size in the range of 60 to 100u, covered thoroughly with Sulfur at lower temperature leSS 2,589,653 3/1952 Alvarez-Tostado et al. ........... 423/344 2,766,103 10/1956 Nielsen et al. .......................... 423/344 than 700 C. in vacuum. In order to produce the silicon 3,321,326 5/1967 Young ............. -

{Download PDF}
NO! PDF, EPUB, EBOOK Marta Altes | 32 pages | 15 May 2012 | Child's Play International Ltd | 9781846434174 | English | Swindon, United Kingdom trình giả lập trên PC và Mac miễn phí – Tải NoxPlayer Don't have an account? Sign up here. Already have an account? Log in here. By creating an account, you agree to the Privacy Policy and the Terms and Policies , and to receive email from Rotten Tomatoes and Fandango. Please enter your email address and we will email you a new password. We want to hear what you have to say but need to verify your account. Just leave us a message here and we will work on getting you verified. No uses its history-driven storyline to offer a bit of smart, darkly funny perspective on modern democracy and human nature. Rate this movie. Oof, that was Rotten. Meh, it passed the time. So Fresh: Absolute Must See! You're almost there! Just confirm how you got your ticket. Cinemark Coming Soon. Regal Coming Soon. By opting to have your ticket verified for this movie, you are allowing us to check the email address associated with your Rotten Tomatoes account against an email address associated with a Fandango ticket purchase for the same movie. Geoffrey Macnab. Gripping and suspenseful even though the ending is already known. Rene Rodriguez. The best movie ever made about Chilean plebiscites, No thoroughly deserves its Oscar nomination for Best Foreign Film. Anthony Lane. Soren Andersen. Calvin Wilson. A cunning and richly enjoyable combination of high-stakes drama and media satire from Chilean director Pablo Larrain. -
Naming Compounds Quiz A
Naming Compounds Quiz A 1. How can you quickly tell if a compound is molecular or ionic? Give an example of each kind. 2. Name these compounds: NaBr, MgS, AlN, Na 2S, Mg 3P2, AlF 3. 3. Name these compounds: FeO, Fe 2O3, WS 3, TiI 4, TiI 3, ZnBr 2. 4. Name these compounds: N 2O, NO 2, N 2O5, CO, CO 2, SeBr 2. 5. Name these compounds: KNO 2, KNO 3, Al(OH) 3, (NH 4)2CrO 4, (NH 4)2Cr 2O7, CaC 2O4. 6. Name these compounds: Cu(NO3) 2, CuNO 3, AlPO 4, Nb(MnO 4)5, Nb 2(SO 3)3, NaCN. 7. List 10 prefixes used to name molecular compounds 1 to 10. 8. What metals use roman numerals? 9. Why don’t we ever say something like sodium (I) chloride, or zinc (II) oxide? 10. Why does EVERY COMPOUND in the Universe with manganese HAVE TO HAVE a roman numeral? What ion charges go with which roman numerals? Naming Compounds Quiz A ANSWERS 1. How can you quickly tell if a compound is molecular or ionic? Give an example of each kind. Molecular compounds always start with nonmetals, ex: CO, H 2O, CO 2, CH 4, C 6H12 O6. +1 Ionic compounds always start with metals, ex: NaCl, MgO, TiBr 2, TiBr 3, TiBr 4 - one exception: NH 4 2. Name these compounds: NaBr, MgS, AlN, Na 2S, Mg 3P2, AlF 3. sodium bromide, magnesium sulfide, aluminium nitride, sodium sulfide, magnesium phosphide, aluminum fluoride. 3. Name these compounds: FeO, Fe 2O3, WS 3, TiI 4, TiI 3, ZnBr 2. -
Rotational Spectrum and Equilibrium Structure of Silanethione, H2siqs
COMMUNICATION www.rsc.org/chemcomm | ChemComm Rotational spectrum and equilibrium structure of silanethione, H2SiQS Sven Thorwirth,*a Ju¨rgen Gauss,b Michael C. McCarthy,c Franc¸ois Shindoc and Patrick Thaddeusc Received (in Cambridge, UK) 20th August 2008, Accepted 23rd September 2008 First published as an Advance Article on the web 1st October 2008 DOI: 10.1039/b814558j 9,14 Unsubstituted silanethione, H2SiQS, has been characterized structures, a search for the fundamental a-type rotational experimentally for the first time by means of rotational spectros- transition JKa,Kc =10,1–00,0 of H2SiQS was performed in a copy; the equilibrium structure of this fundamental molecule has 3 GHz interval near 15.7 GHz. Spectral analysis of lines in this been evaluated through a combination of experimental data from region, however, was hampered by the presence of the corres- a total of ten isotopologues and results of high-level coupled- ponding J = 1–0 rotational lines and accompanying vibra- 8 cluster calculations. tional satellites of SiS and its isotopic species. Replacing SiH4 and H2S with SiD4 and D2S revealed that only a moderate Silanethiones, RR0SiQS, the second-row element analogues to number of lines were from molecules that simultaneously ketones, have received considerable attention over the past contained sulfur, silicon, and hydrogen. To rule out radicals, two decades through studies of multiple bonding between unidentified lines with the required elemental composition group 14 and group 16 elements.1 Several silanethiones stabi- were tested for Zeeman splitting in the presence of an external lized by bulky substituents were synthesized and their struc- magnetic field (with a permanent magnet mounted near the tures characterized using X-ray crystallography.1,2 Infrared molecular beam). -
List of Molecules and Ions with 2 to 10 Atoms
List of Molecules and Ions with 2 to 10 Atoms Diatomic (44) Molecule Designation Mass Ions AlCl Aluminium monochloride [39] [40] 62.5 — AlF Aluminium monofluoride [39] [41] 46 — AlO Aluminium monoxide [42] 43 — — Argonium [43] [44] 41 ArH+ C2 Diatomic carbon [45] [46] 24 — — Fluoromethylidynium 31 CF+[47] CH Methylidyne radical [32] [48] 13 CH+[49] CN Cyanogen radical [39] [48] [50] [51] 26 CN+,[52] CN−[53] CO Carbon monoxide [39] [54] [55] 28 CO+[56] CP Carbon monophosphide [51] 43 — CS Carbon monosulfide [39] 44 — FeO Iron(II) oxide [57] 82 — — Helium hydride ion [58] [59] 5 HeH+ H2 Molecular hydrogen [60] 2 — HCl Hydrogen chloride [61] 36.5 HCl+[62] HF Hydrogen fluoride [63] 20 — HO Hydroxyl radical [39] 17 OH+[64] KCl Potassium chloride [39] [40] 75.5 — NH Nitrogen monohydride [65] [66] 15 — N2 Molecular nitrogen [67] [68] 28 — NO Nitric oxide [69] 30 NO+[52] NS Nitrogen sulfide [39] 46 — NaCl Sodium chloride [39] [40] 58.5 — — Magnesium monohydride cation 25.3 MgH+[52] NaI Sodium iodide [70] 150 — O2 Molecular oxygen [71] 32 — PN Phosphorus mononitride [72] 45 — PO Phosphorus monoxide [73] 47 — SH Sulfur monohydride [74] 33 SH+[75] SO Sulfur monoxide [39] 48 SO+[49] SiC Carborundum [39] [76] 40 — SiN Silicon mononitride [39] 42 — SiO Silicon monoxide [39] 44 — Molecule Designation Mass Ions SiS Silicon monosulfide [39] 60 — TiO Titanium oxide [77] 63.9 — The H+ 3 cation is one of the most abundant ions in the universe. It was first detected in 1993. [78] [79] Triatomic (41) Molecule Designation Mass Ions AlNC Aluminium isocyanide -

In the Interstellar Medium, Cosmic Rays Comprised Mostly of High-Energy
In the interstellar medium, cosmic rays comprised mostly of high-energy protons can drive fast non-thermal reactions in the ice mantles covering microscopic dust grains. Illustrated here is such a collision, highlighting the tracks (shown in red) of both the cosmic ray and "secondary electrons" that are formed in the ice. This track was generated using the CIRIS program (Shingledecker et al., 2017). ON COSMIC RAYS IN ASTROCHEMICAL MODELS Christopher Nelson Shingledecker Virginia Beach, Virginia B.Sc. Chemistry with Highest Distinction University of Virginia, 2013 A Dissertation Presented to the Graduate Faculty of the University of Virginia in Candidacy for the Degree of Doctor of Philosophy Department of Chemistry University of Virginia April 2018 ii ⃝c Copyright by Christopher Nelson Shingledecker All rights reserved May 12, 2018 iii ABSTRACT OSMIC rays are known to have significant physicochemical impact on interstellar regions (Indriolo and McCall, 2013). For example, even in the very first astro- chemical modeling work of Herbst and Klemperer (1973), cosmic rays were shown to drive the gas-phase chemistry of molecular clouds through the ion- + Cization of H2 and subsequent formation of H3 . Later, Prasad and Tarafdar (1983) demon- strated that cosmic rays - and the secondary electrons they generate - could collisionally excite the Lyman and Werner bands of H2, leading to the formation of internal UV pho- tons in dense regions where the external interstellar UV radiation field is quickly attenu- ated. However, despite the central role that the ionization and excitation of H2 currently plays in astrochemical models, extending the treatment of these types of interactions to other species has proven challenging thanks to both the complexity and variety of under- lying microscopic processes, which has stymied the development of suitable theoretical and computational techniques. -
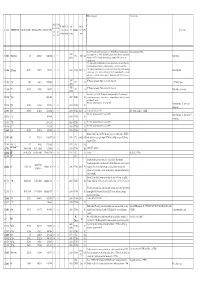
Molec Tab0410 Pub
priority for measurements ? Extra info spectro extra info astro J Max J Min K Max K Min Last lowest meas. meas. # TAG MOLECULE Min.Meas.(MHz) Max Meas.(MHz) Max Pred.(MHz) meas. meas. M. Database v level QNF Species name (or (or (or Ka) (or Ka) (entry) cm-1 N) N) The latest JPL entry includes predictions for v = 1, but otherwise appears highly similar to Detected (in list CDMS). 2007 2 13002 CH (v=0,1) 701 4250352 14289108 5 1 JPL 2733 224 that previously created in CDMS (13502) which gives predictions (for v=0 only) up to higher Methylidyne (2010) frequency (14.3 THz). Keywords: lamda-doubling, spin-rotation, LMR, infrared, not particularly rigid, * A combined fit of all available data of all isotopic species has been carried out. Note that the rotational transition measured is at different frequnency to that of previous entries. Additional rovibrational transitions between the electronic X and A states of four isotopologs 2 13503 CH+ (ion) 835137 835137 7398193 1 0 2010 CDMS 2753.6 101 Methylidynium were also used. There are two lines in the range of HIFI. Predictions should be viewed with some caution; considerable caution is advised for transitions beyond J" = 10. Keyword: ab- inito dipole moment 2 13 2008 X Π states, keywords: Hunds case(b), fine, hyperfine 2 14003 CH 7091 536132 14789069 2 1 JPL 255 13 C-Methylidyne (2009) 2008 2 2 14004 CD 439255 916954 1862237 2 1 JPL 234 X Π states, keywords: Hunds case(b), fine, hyperfine Methylidyne, deuterated (2009) New entry.