Complement Component 5 Contributes to Poor Disease Outcome in Humans and Mice with Pneumococcal Meningitis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Important Roles of C5a and C5ar in Tumor Development and Cancer Treatment
E3S Web o f Conferences 136, 06012 (2019) https://doi.org/10.1051/e3sconf/20191360 6012 ICBTE 2019 Important roles of C5a and C5aR in tumor development and cancer treatment Wang Yuxuan Bioengineering, School of Life Science, Anhui University,230601 Abstract: The complement system is part of the body's innate defense immune system, which can identify and eliminate invasive pathogenic microorganisms to maintain normal life activities. Complement Component 5a (C5a) is an active anaphylatoxin produced after complement system activation, closely related to tumor formation. C5a is highly expressed in a variety of tumors, and combines with its Complement Component 5a Receptor (C5aR) to increase the proliferation and migration of tumor cells. This review will comprehensively elaborate the important role of C5a/C5aR in the process of tumor genesis and development from the three aspects of signal transduction pathways related to tumor, C5a/C5aR and tumor formation, and C5a/C5aR inhibitors and tumor therapy. Finally, the principle of complement inhibition is used to inhibit tumor metastasis, reduce the rate of tumor diffusion, and control the trend of tumor deterioration. 1 Introduction The generation of tumor is closely related to the microenvironment in which tumor is located. A variety of cells in the microenvironment, such as lymphocytes, macrophages, fibroblasts and various molecules, such as complement and cytokines, are involved in the survival, proliferation and metastasis of tumor cells. Complement Component is a protein with enzyme activity. Since the discovery of complement system by Belgian doctor J. Bordet in 1890, a large number of studies have shown that complement exists in human serum, tissue fluid and cell membrane surface1. -

Shiga Toxin 2A Binds to Complement Components C3b and C5 and Upregulates Their Gene Expression in Human Cell Lines
toxins Article Shiga Toxin 2a Binds to Complement Components C3b and C5 and Upregulates Their Gene Expression in Human Cell Lines Sára Kellnerová 1,†, Sneha Chatterjee 1,†, Rafael Bayarri-Olmos 2 , Louise Justesen 2 , Heribert Talasz 3, Wilfried Posch 1 , Samyr Kenno 1, Peter Garred 2, Dorothea Orth-Höller 1,*, Marco Grasse 1 and Reinhard Würzner 1,* 1 Institute of Hygiene and Medical Microbiology, Medical University of Innsbruck, Schöpfstraβe 41, A-6020 Innsbruck, Austria; [email protected] (S.K.); [email protected] (S.C.); [email protected] (W.P.); [email protected] (S.K.); [email protected] (M.G.) 2 Laboratory of Molecular Medicine, Department of Clinical Immunology Section 7631, Rigshospitalet, University of Copenhagen, Ole Maaloesvej 26, 2200 Copenhagen, Denmark; [email protected] (R.B.-O.); [email protected] (L.J.); [email protected] (P.G.) 3 Centre of Chemistry and Biomedicine, Division of Clinical Biochemistry, Medical University of Innsbruck, Innrain 80, A-6020 Innsbruck, Austria; [email protected] * Correspondence: [email protected] (D.O.-H.); [email protected] (R.W.); Tel.: +43-512-900-370-707 (R.W.) † First authors. Abstract: Enterohemorrhagic Escherichia coli (EHEC) infections can cause EHEC-associated hemolytic uremic syndrome (eHUS) via its main virulent factor, Shiga toxins (Stxs). Complement has been reported to be involved in the progression of eHUS. The aim of this study was to investigate the interactions of the most effective subtype of the toxin, Stx2a, with pivotal complement proteins C3b and C5. -
Review Article Complement System and Age-Related Macular Degeneration: Implications of Gene-Environment Interaction for Preventive and Personalized Medicine
Hindawi BioMed Research International Volume 2018, Article ID 7532507, 13 pages https://doi.org/10.1155/2018/7532507 Review Article Complement System and Age-Related Macular Degeneration: Implications of Gene-Environment Interaction for Preventive and Personalized Medicine Andrea Maugeri ,1 Martina Barchitta ,1 Maria Grazia Mazzone,2 Francesco Giuliano,2 and Antonella Agodi 1 1 Department of Medical and Surgical Sciences and Advanced Technologies “GF Ingrassia”, University of Catania, Via S. Sofa 87, 95123 Catania, Italy 2SIFI SpA, Research and Development Department, Via Ercole Patti 36, 95025 Catania, Italy Correspondence should be addressed to Antonella Agodi; [email protected] Received 18 May 2018; Accepted 18 July 2018; Published 26 August 2018 Academic Editor: Sajib Chakraborty Copyright © 2018 Andrea Maugeri et al. Tis is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Age-related macular degeneration (AMD) is the most common cause of visual loss in developed countries, with a signifcant economic and social burden on public health. Although genome-wide and gene-candidate studies have been enabled to identify genetic variants in the complement system associated with AMD pathogenesis, the efect of gene-environment interaction is still under debate. In this review we provide an overview of the role of complement system and its genetic variants in AMD, summarizing the consequences of the interaction between genetic and environmental risk factors on AMD onset, progression, and therapeutic response. Finally, we discuss the perspectives of current evidence in the feld of genomics driven personalized medicine and public health. -

High-Throughput Proteomic Profiling of the Fish Liver Following Bacterial
Causey et al. BMC Genomics (2018) 19:719 https://doi.org/10.1186/s12864-018-5092-0 RESEARCH ARTICLE Open Access High-throughput proteomic profiling of the fish liver following bacterial infection Dwight R Causey1, Moritz A N Pohl1, David A Stead2, Samuel A M Martin1, Christopher J Secombes1 and Daniel J Macqueen1* Abstract Background: High-throughput proteomics was used to determine the role of the fish liver in defense responses to bacterial infection. This was done using a rainbow trout (Oncorhynchus mykiss) model following infection with Aeromonas salmonicida, the causative agent of furunculosis. The vertebrate liver has multifaceted functions in innate immunity, metabolism, and growth; we hypothesize this tissue serves a dual role in supporting host defense in parallel to metabolic adjustments that promote effectiveimmunefunction.Whilepaststudieshavereported mRNA responses to A. salmonicida in salmonids, the impact of bacterial infection on the liver proteome remains uncharacterized in fish. Results: Rainbow trout were injected with A. salmonicida or PBS (control) and liver extracted 48 h later for analysis on a hybrid quadrupole-Orbitrap mass spectrometer. A label-free method was used for protein abundance profiling, which revealed a strong innate immune response along with evidence to support parallel rewiring of metabolic and growth systems. 3076 proteins were initially identified against all proteins (n = 71,293 RefSeq proteins) annotated in a single high-quality rainbow trout reference genome, of which 2433 were maintained for analysis post-quality filtering. Among the 2433 proteins, 109 showed significant differential abundance following A. salmonicida challenge, including many upregulated complement system and acute phase response proteins, in addition to molecules with putative functions that may support metabolic re-adjustments. -

Are Complement Deficiencies Really Rare?
G Model MIMM-4432; No. of Pages 8 ARTICLE IN PRESS Molecular Immunology xxx (2014) xxx–xxx Contents lists available at ScienceDirect Molecular Immunology j ournal homepage: www.elsevier.com/locate/molimm Review Are complement deficiencies really rare? Overview on prevalence, ଝ clinical importance and modern diagnostic approach a,∗ b Anete Sevciovic Grumach , Michael Kirschfink a Faculty of Medicine ABC, Santo Andre, SP, Brazil b Institute of Immunology, University of Heidelberg, Heidelberg, Germany a r a t b i c s t l e i n f o r a c t Article history: Complement deficiencies comprise between 1 and 10% of all primary immunodeficiencies (PIDs) accord- Received 29 May 2014 ing to national and supranational registries. They are still considered rare and even of less clinical Received in revised form 18 June 2014 importance. This not only reflects (as in all PIDs) a great lack of awareness among clinicians and gen- Accepted 23 June 2014 eral practitioners but is also due to the fact that only few centers worldwide provide a comprehensive Available online xxx laboratory complement analysis. To enable early identification, our aim is to present warning signs for complement deficiencies and recommendations for diagnostic approach. The genetic deficiency of any Keywords: early component of the classical pathway (C1q, C1r/s, C2, C4) is often associated with autoimmune dis- Complement deficiencies eases whereas individuals, deficient of properdin or of the terminal pathway components (C5 to C9), are Warning signs Prevalence highly susceptible to meningococcal disease. Deficiency of C1 Inhibitor (hereditary angioedema, HAE) Meningitis results in episodic angioedema, which in a considerable number of patients with identical symptoms Infections also occurs in factor XII mutations. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Practice Parameter for the Diagnosis and Management of Primary Immunodeficiency
Practice parameter Practice parameter for the diagnosis and management of primary immunodeficiency Francisco A. Bonilla, MD, PhD, David A. Khan, MD, Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD, David I. Bernstein, MD, Joann Blessing-Moore, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Chief Editor: Francisco A. Bonilla, MD, PhD Co-Editor: David A. Khan, MD Members of the Joint Task Force on Practice Parameters: David I. Bernstein, MD, Joann Blessing-Moore, MD, David Khan, MD, David Lang, MD, Richard A. Nicklas, MD, John Oppenheimer, MD, Jay M. Portnoy, MD, Christopher R. Randolph, MD, Diane Schuller, MD, Sheldon L. Spector, MD, Stephen Tilles, MD, Dana Wallace, MD Primary Immunodeficiency Workgroup: Chairman: Francisco A. Bonilla, MD, PhD Members: Zuhair K. Ballas, MD, Javier Chinen, MD, PhD, Michael M. Frank, MD, Joyce T. Hsu, MD, Michael Keller, MD, Lisa J. Kobrynski, MD, Hirsh D. Komarow, MD, Bruce Mazer, MD, Robert P. Nelson, Jr, MD, Jordan S. Orange, MD, PhD, John M. Routes, MD, William T. Shearer, MD, PhD, Ricardo U. Sorensen, MD, James W. Verbsky, MD, PhD GlaxoSmithKline, Merck, and Aerocrine; has received payment for lectures from Genentech/ These parameters were developed by the Joint Task Force on Practice Parameters, representing Novartis, GlaxoSmithKline, and Merck; and has received research support from Genentech/ the American Academy of Allergy, Asthma & Immunology; the American College of Novartis and Merck. -

Uva-DARE (Digital Academic Repository)
UvA-DARE (Digital Academic Repository) Flow cytometric immunophenotyping in the diagnosis and follow-up of immunodeficient children de Vries, E.; Noordzij, J.G.; Kuijpers, T.W.; van Dongen, J.J.M. DOI 10.1007/s004310100797 Publication date 2001 Published in European Journal of Pediatrics Link to publication Citation for published version (APA): de Vries, E., Noordzij, J. G., Kuijpers, T. W., & van Dongen, J. J. M. (2001). Flow cytometric immunophenotyping in the diagnosis and follow-up of immunodeficient children. European Journal of Pediatrics, 160(10), 583-591. https://doi.org/10.1007/s004310100797 General rights It is not permitted to download or to forward/distribute the text or part of it without the consent of the author(s) and/or copyright holder(s), other than for strictly personal, individual use, unless the work is under an open content license (like Creative Commons). Disclaimer/Complaints regulations If you believe that digital publication of certain material infringes any of your rights or (privacy) interests, please let the Library know, stating your reasons. In case of a legitimate complaint, the Library will make the material inaccessible and/or remove it from the website. Please Ask the Library: https://uba.uva.nl/en/contact, or a letter to: Library of the University of Amsterdam, Secretariat, Singel 425, 1012 WP Amsterdam, The Netherlands. You will be contacted as soon as possible. UvA-DARE is a service provided by the library of the University of Amsterdam (https://dare.uva.nl) Download date:25 Sep 2021 Eur J Pediatr -2001) 160: 583±591 DOI 10.1007/s004310100797 REVIEW Esther de Vries á Jeroen G. -

Complement and Inflammasome Overactivation Mediates Paroxysmal Nocturnal Hemoglobinuria with Autoinflammation
Complement and inflammasome overactivation mediates paroxysmal nocturnal hemoglobinuria with autoinflammation Britta Höchsmann, … , Peter M. Krawitz, Taroh Kinoshita J Clin Invest. 2019. https://doi.org/10.1172/JCI123501. Research Article Hematology Inflammation Graphical abstract Find the latest version: https://jci.me/123501/pdf The Journal of Clinical Investigation RESEARCH ARTICLE Complement and inflammasome overactivation mediates paroxysmal nocturnal hemoglobinuria with autoinflammation Britta Höchsmann,1,2 Yoshiko Murakami,3,4 Makiko Osato,3,5 Alexej Knaus,6 Michi Kawamoto,7 Norimitsu Inoue,8 Tetsuya Hirata,3 Shogo Murata,3,9 Markus Anliker,1 Thomas Eggermann,10 Marten Jäger,11 Ricarda Floettmann,11 Alexander Höllein,12 Sho Murase,7 Yasutaka Ueda,5 Jun-ichi Nishimura,5 Yuzuru Kanakura,5 Nobuo Kohara,7 Hubert Schrezenmeier,1 Peter M. Krawitz,6 and Taroh Kinoshita3,4 1Institute of Transfusion Medicine, University of Ulm, Ulm, Germany. 2Institute of Clinical Transfusion Medicine and Immunogenetics, German Red Cross Blood Transfusion Service and University Hospital Ulm, Ulm, Germany. 3Research Institute for Microbial Diseases and 4WPI Immunology Frontier Research Center, Osaka University, Osaka, Japan. 5Department of Hematology and Oncology, Graduate School of Medicine, Osaka University, Osaka, Japan. 6Institute for Genomic Statistics and Bioinformatics, Rheinische Friedrich-Wilhelms-Universität Bonn, Bonn, Germany. 7Department of Neurology, Kobe City Medical Center General Hospital, Kobe, Japan. 8Department of Tumor Immunology, Osaka -
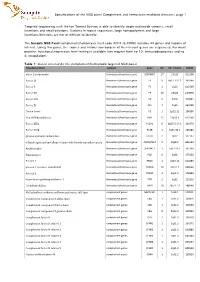
Specifications of the NGS Panel Complement and Hemostasis Mediated Diseases| Page 1
Specifications of the NGS panel Complement and hemostasis mediated diseases| page 1 Targeted sequencing with the Ion Torrent System is able to identify single nucleotide variants, small insertions and small deletions. Variants in repeat sequences, large homopolymers and large insertions/deletions are not or difficult to identify. The Sanquin NGS Panel complement/hemostasis (test code X001 to X006) includes 44 genes and regions of interest. Using this panel, the exones and intron/exon borders of the relevant genes are sequenced. For most proteins, functional/expression level testing is available (see request form no 10: immunodiagnostics and no 4: coagulation). Table 1: Genes covered by the complement/hemostasis targeted NGS panel. Encoded protein Context Gene Chr Chr. Positie OMIM alpha 2-antiplasmin Hemostasis/trombosis gene SERPINF2 17 17p13 613168 Factor IX Hemostasis/trombosis gene F9 X Xq27.1-27.2 300746 Factor V Hemostasis/trombosis gene F5 1 1q23 612309 Factor VII Hemostasis/trombosis gene F7 13 13q34 613878 Factor VIII Hemostasis/trombosis gene F8 X Xq28 300841 Factor XI Hemostasis/trombosis gene F11 4 4q35 264900 Tissue factor Hemostasis/trombosis gene F3 1 1p22-21 134390 Von Willebrand factor Hemostasis/trombosis gene VWF 12 12p13.3 613160 Factor XIIIa Hemostasis/trombosis gene F13A1 6 6p25.3-24.3 134570 Factor XIIIb Hemostasis/trombosis gene F13B 1 1q31-32.1 134580 gamma-glutamyl carboxylase Hemostasis/trombosis gene GGCX 2 2p12 137167 a Disintergrin and metalloproteinase wih thrombospondin repeats Hemostasis/trombosis gene -

European Society for Immunodeficiencies (ESID)
Journal of Clinical Immunology https://doi.org/10.1007/s10875-020-00754-1 ORIGINAL ARTICLE European Society for Immunodeficiencies (ESID) and European Reference Network on Rare Primary Immunodeficiency, Autoinflammatory and Autoimmune Diseases (ERN RITA) Complement Guideline: Deficiencies, Diagnosis, and Management Nicholas Brodszki1 & Ashley Frazer-Abel2 & Anete S. Grumach3 & Michael Kirschfink4 & Jiri Litzman5 & Elena Perez6 & Mikko R. J. Seppänen7 & Kathleen E. Sullivan8 & Stephen Jolles9 Received: 5 June 2019 /Accepted: 20 January 2020 # The Author(s) 2020 Abstract This guideline aims to describe the complement system and the functions of the constituent pathways, with particular focus on primary immunodeficiencies (PIDs) and their diagnosis and management. The complement system is a crucial part of the innate immune system, with multiple membrane-bound and soluble components. There are three distinct enzymatic cascade pathways within the complement system, the classical, alternative and lectin pathways, which converge with the cleavage of central C3. Complement deficiencies account for ~5% of PIDs. The clinical consequences of inherited defects in the complement system are protean and include increased susceptibility to infection, autoimmune diseases (e.g., systemic lupus erythematosus), age-related macular degeneration, renal disorders (e.g., atypical hemolytic uremic syndrome) and angioedema. Modern complement analysis allows an in-depth insight into the functional and molecular basis of nearly all complement deficiencies. However, therapeutic options remain relatively limited for the majority of complement deficiencies with the exception of hereditary angioedema and inhibition of an overactivated complement system in regulation defects. Current management strategies for complement disor- ders associated with infection include education, family testing, vaccinations, antibiotics and emergency planning. Keywords Complement . -

International Union of Basic and Clinical Pharmacology. LXXXVII
Supplemental Material can be found at: /content/suppl/2014/09/18/65.1.500.DC1.html 1521-0081/65/1/500–543$25.00 http://dx.doi.org/10.1124/pr.111.005223 PHARMACOLOGICAL REVIEWS Pharmacol Rev 65:500–543, January 2013 Copyright © 2013 by The American Society for Pharmacology and Experimental Therapeutics International Union of Basic and Clinical Pharmacology. LXXXVII. Complement Peptide C5a, C4a, and C3a Receptors Andreas Klos, Elisabeth Wende, Kathryn J. Wareham, and Peter N. Monk Department for Medical Microbiology, Medical School Hannover, Hannover, Germany (A.K., E.W.); and the Department of Infection and Immunity, University of Sheffield Medical School, Sheffield, United Kingdom (K.J.W., P.N.M.) Abstract. ....................................................................................502 I. Introduction. ..............................................................................503 A. Production of Complement Peptides......................................................503 B. Concentrations of Complement Peptides in Health and Disease. .........................503 C. C3a and C5a Generation outside the Complement Cascade ...............................504 D. Deactivation of Complement Peptides ....................................................505 II. The Role of Complement Peptides in Pathophysiology . .....................................505 A. Complement Peptides Are Important Biomarkers of Disease..............................505 B. Functions of the Complement Peptides beyond Innate Immunity .........................506