History of Metallurgy
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Iron-Making Process
Hopewell Furnace National Historic Site www.nps.gov/hofu Teacher’s Guide The Iron-Making Process The Ingredients The four main ingredients for making iron were present in the area of Hopewell and were a reason for the furnace’s location. I. Wood A. Used as a primary energy source in village operations 1. As charcoal to power the furnace and blacksmith shop 2. As fuel for heating and cooking B. Used as building material for: 1. Homes 2. Wagons and equipment II. Water A. Used to turn the water wheel connected to the bellows which forced blasts of air into the furnace to increase the heat B. Used for: 1. Drinking 2. Bathing 3. Cooking 4. Washing 5. Cleaning III. Iron Ore Iron ore was dug from open-pit mines near Hopewell, then hauled to Hopewell Furnace in carts and wagons IV. Limestone Used as a “flux,” which removed impurities from the ore as the iron ore was smelted; it was quarried near Morgantown and from Hopewell Plantation lands The Process This process was also called smelting. I. In the furnace, the charcoal burns with a great amount of heat. II. The water wheel was attached to a huge bellows and later to blowing tubs — like can be seen at Hopewell today — which blasted the charcoal with air to increase the heat. III. The furnace was filled, or “charged,” from the top in this order: A. Fire B. Charcoal Iron-Making Process Page 1 C. Iron Ore D. Limestone E. Charcoal F. Iron Ore G. The process continued and repeated as the ore melted IV. -

Two Steps Forward, One Step Back: a Brief History of Corporate Citizenship and Corporate Social Responsibility
Two Steps Forward, One Step Back: A Brief History of Corporate Citizenship and Corporate Social Responsibility Stephen Jordan with B.J. Parker BCLC’s mission is to promote better business and society relations and improve long-term social and economic conditions by: • Communicating the U.S. private sector’s unique and valuable contributions • Cultivating strategies and practices that achieve positive results • Coordinating public-private partnerships and coalitions 2 TABLE OF CONTENTS CHAPTER ONE: DEFINING CORPORATE CITIZENSHIP AND CORPORATE SOCIAL RESPONSIBILITY . .1 CHAPTER TWO: THE ORIGINS OF CAPITALISM AND CORPORATE CITIZENSHIP . .6 CHAPTER THREE: THE RISE OF THE INDUSTRIAL REVOLUTION AND PATERNALISM . 9 CHAPTER FOUR: THE GILDED AGE AND PERSONAL PHILANTHROPY . .12 CHAPTER FIVE: THE FIRST ETHICAL CORPORATIONS . 15 CHAPTER SIX: THE COMMUNITY CHEST MOVEMENT, THE GREAT DEPRESSION, AND WORLD WAR II . 20 CHAPTER SEVEN: THE 1950s - 1970s . .26 CHAPTER EIGHT: 1980 AND THE DAWN OF THE MODERN CORPORATE CITIZENSHIP MOVEMENT . 31 CHAPTER NINE: THE VIEW FROM OUTSIDE . .35 CHAPTER TEN: THE CONTINUING EVOLUTION OF CORPORATE CITIZENSHIP . 41 CHAPTER ELEVEN: DIFFERENT WAYS THE HISTORY OF CORPORATE CITIZENSHIP COULD BE TOLD . 46 Cover photos (clockwise): Andrew Carnegie, Henry Ford, Indra Nooyi (courtesy of PepsiCo), Thomas J. Watson (courtesy of IBM), Robert Wood Johnson II, Bill and Melinda Gates (Wikipedia Commons User Kjetil Ree). About the Authors Stephen Jordan is the founder and executive director of the Business Civic Leadership Center (BCLC), the corporate citizenship affiliate of the U.S. Chamber of Commerce. He is married and has two children. B.J. Parker is a professional writer and editor with more than 10 years’ experience writing business education materials for leading educational publishers. -

2000 Stainless Steels: an Introduction to Their Metallurgy and Corrosion
Dairy, Food and Environmental Sanitation, Vol. 20, No. 7, Pages 506-517 Copyright© International Association for Food Protection, 6200 Aurora Ave., Suite 200W, Des Moines, IA 50322 Stainless Steels: An Introduction to Their Metallurgy and Corrosion Resistance Roger A. Covert and Arthur H. Tuthill* and why they sometimes do not. In most cases, selection of the proper stainless steel leads to satisfactory performance. COMPOSITION, NOMEN- CLATURE AND GENERAL PROPERTIES Most metals are mixtures of a primary metallic element and one or more intentionally added other ele- This article has been peer-reviewed by two professionals. ments. These mixtures of elements are called alloys. Stainless steels are alloys, as are brasses (copper + zinc), bronzes (copper + tin), the many alu- INTRODUCTION better understanding of stainless minum alloys, and many other me- Worldwide, in industry, in busi- steels, especially to the non-metal- tallic materials. In general, solid ness and in the home, metals called lurgist. metals and alloys consist of randomly stainless steels are used daily. It is Industries are concerned with oriented grains that have a well-de- important to understand what these integrity of equipment and product fined crystalline structure, or lattice, materials are and why they behave purity. To achieve these, stainless within the grains. In stainless steels, the way they do. This is especially steels are often the economical and the crystalline structures within the true because the word “stainless” is practical materials of choice for pro- grains have been given names such as itself somewhat of a misnomer; these cess equipment. However, before ferrite, austenite, martensite, or a materials can stain and can corrode intelligent decisions can be made mixture of two or more of these. -

National Register of Historic Places Multiple Property
NFS Form 10-900-b 0MB No. 1024-0018 (Jan. 1987) United States Department of the Interior National Park Service National Register of Historic Places Multipler Propertyr ' Documentation Form NATIONAL This form is for use in documenting multiple property groups relating to one or several historic contexts. See instructions in Guidelines for Completing National Register Forms (National Register Bulletin 16). Complete each item by marking "x" in the appropriate box or by entering the requested information. For additional space use continuation sheets (Form 10-900-a). Type all entries. A. Name of Multiple Property Listing ____Iron and Steel Resources of Pennsylvania, 1716-1945_______________ B. Associated Historic Contexts_____________________________ ~ ___Pennsylvania Iron and Steel Industry. 1716-1945_________________ C. Geographical Data Commonwealth of Pennsylvania continuation sheet D. Certification As the designated authority under the National Historic Preservation Act of 1966, as amended, J hereby certify that this documentation form meets the National Register documentation standards and sets forth requirements for the listing of related properties consistent with the National Register criteria. This submission meets the procedural and professional requiremerytS\set forth iri36JCFR PafrfsBOfcyid the Secretary of the Interior's Standards for Planning and Evaluation. Signature of certifying official Date / Brent D. Glass Pennsylvania Historical & Museum Commission State or Federal agency and bureau I, hereby, certify that this multiple -

The Neolithic Copper Melting Crucibles from Switzerland
In: A. Shortland, I. Freestone and Th. FromRehren mine (eds) to microbe 2009, From Mine to Microscope, 155-162 155 Chapter 15 From mine to microbe – the Neolithic copper melting crucibles from Switzerland Th. Rehren1 Abstract The occurrence of chalcopyrite in several late Neolithic crucibles from NW Switzerland and SW Germany has been variously interpreted as indicating evidence for local copper smelting, or being due to post-depositional phenomena. This study uses optical microscopy and a discussion based on textural and micro-stratigraphical arguments to demonstrate that chalcopyrite is a late formation and not indicative of copper smelting. This has significant implications for the technological and archaeological interpetation of these finds, but also illustrates the potential of image-based studies in science-based archaeology. Introduction these cultures back into line with their neighbours. The emergence and spread of metallurgy in Europe Only after a further half a millennium or so metal- is a major concern of archaeological and archaeo- lurgy emerges again in Central Europe, heralding the metallurgical research. In the 1960s scholars such as beginning of the Bronze Age throughout the Contin- Renfrew and Branigan focused their attention – and ent. This time, it is a broad and sustained develop- consequently that of others – on the role which metals ment with no particular emphasis on the Swiss or have played in the development of stratified societies southwest German regions. and the emergence of elites, showing off with their It is against this background that the crucible access to these new materials. The Balkans and fragments of the Pfyn culture have attracted attention Western Asia are now both known to have had for more than a century, having been found at a wide metallurgically competent Neolithic cultures, and range of sites and representing certainly several issues of technology transfer and autochthonous dozen different vessels. -

The Evolution of Engineering Materials
Paper ID #20611 The Evolution of Engineering Materials Dr. Amber L. Genau, University of Alabama at Birmingham Dr. Amber Genau is an assistant professor in the Materials Science and Engineering Department at the University of Alabama at Birmingham. She received her B.S. and M.S. from Iowa State University and Ph.D. from Northwestern University, all in materials engineering. Before coming to UAB, Dr. Genau spent two years as a guest scientist at the German Aerospace Center in Cologne, Germany, working on metal solidification and microstructural characterization. She is particularly interested in broadening participation in engineering and providing international experiences and perspectives to undergraduate students. c American Society for Engineering Education, 2017 The Evolution of Engineering Materials Abstract This paper describes the development of an upper level engineering elective entitled “The Evolution of Engineering Materials.” The course considers how the discovery of new materials and the ability of process materials in new ways has influenced the course of history, shaping both human societies and their surrounding environments, from the Stone Age to the Modern Era. Students become familiar with a variety of still-relevant technical content through the consideration of historical activity, from smelting and coking to polymerization reactions and cross linking. The course addresses a variety of ABET outcomes while also supporting the development of global competency through an increased appreciation of world history. Although this course was developed for and taught in the context of a three-week study abroad trip to Europe, the engaging and accessible nature of the content could also make it valuable as a service course for non-engineering majors. -

Comparative Properties of Wrought Iron Made by Hand Puddling and by the Aston Process
RP124 COMPARATIVE PROPERTIES OF WROUGHT IRON MADE BY HAND PUDDLING AND BY THE ASTON PROCESS By Henry S. Rawdon and 0. A. Knight ABSTRACT The hand-puddling method of making wrought iron has not greatly changed for a century. More economical methods in the manufacture of thjs product is the crying need of the industry. A radically new process, recently developed, is now coming into commercial use, in which pig iron, which h>as been refined in a Bessemer converter, is poured into molten slag so as to produce intimate mingling of the two. A comparison of the properties of wrought iron made thus with that made by hand puddling forms the subject of this report. The test results failed to show any marked difference in the products of the two processes. The new product appears to have all of the essential properties usually connoted by the name—wrought iron, CONTENTS Page I. Introduction 954 1. Resume of the Aston process 955 II. Purpose and scope of the investigation 959 III. Materials and methods 960 1. Materials 960 (a) Pipe 960 (6) "Rounds" 961 (c) Slag 962 2. Methods 962 IV. Results 962 1. Composition 962 2. Density 964 3. Mechanical properties 965 (a) Pipe materials 965 (1) Tensile properties 965 (2) Torsional properties 970 (3) Flattening tests 971 (6) 1-inch rounds 972 (1) Tensile properties 972 (2) Torsional properties 973 (3) Impact resistance 973 4. Corrosion resistance 976 (a) Laboratory corrosion tests 976 (6) Electrolytic solution potential 979 5. Structural examination 979 (a) Pipe materials 980 (1) BaU 980 (2) Muck bar 980 (3) Skelp 981 (4) Pipe 981 (&) 1-inch rounds 981 (c) Slag 981 953 : 954 Bureau of Standards Journal of Research [vol. -

Section 1 – Oxygen: the Gas That Changed Everything
MYSTERY OF MATTER: SEARCH FOR THE ELEMENTS 1. Oxygen: The Gas that Changed Everything CHAPTER 1: What is the World Made Of? Alignment with the NRC’s National Science Education Standards B: Physical Science Structure and Properties of Matter: An element is composed of a single type of atom. G: History and Nature of Science Nature of Scientific Knowledge Because all scientific ideas depend on experimental and observational confirmation, all scientific knowledge is, in principle, subject to change as new evidence becomes available. … In situations where information is still fragmentary, it is normal for scientific ideas to be incomplete, but this is also where the opportunity for making advances may be greatest. Alignment with the Next Generation Science Standards Science and Engineering Practices 1. Asking Questions and Defining Problems Ask questions that arise from examining models or a theory, to clarify and/or seek additional information and relationships. Re-enactment: In a dank alchemist's laboratory, a white-bearded man works amidst a clutter of Notes from the Field: vessels, bellows and furnaces. I used this section of the program to introduce my students to the concept of atoms. It’s a NARR: One night in 1669, a German alchemist named Hennig Brandt was searching, as he did more concrete way to get into the atomic every night, for a way to make gold. theory. Brandt lifts a flask of yellow liquid and inspects it. Notes from the Field: Humor is a great way to engage my students. NARR: For some time, Brandt had focused his research on urine. He was certain the Even though they might find a scientist "golden stream" held the key. -

BULLETIN for the HISTORY of CHEMISTRY Division of the History of Chemistry of the American Chemical Society
BULLETIN FOR THE HISTORY OF CHEMISTRY Division of the History of Chemistry of the American Chemical Society NUMBER 2 FALL 1988 eOOH c.,,. - =--- '\ -\.A-'-- - -' II Charles Frederick Chandler (1836 - 1925) Bull. Hist. Chern. 2 (1988) II BULLETIN FOR THE HISTORY OF CHEMISTRY, NO.2, 1988 Editor ............................... WilliamB.Jensen CONTENTS Editorial Assistant ................... Kathy Bailey The BULLETIN FOR THE HISTORY From the Editor's Desk 3 OF CHEMIS1RY is published twice a year by the Division of the History of Letters 3 Chemistry of the American Chemical Society and incorporates the Division's Questions and Queries 3 Newsletter. All changes of address should Do you have a dream? be sent to the current Secretary-Treasurer. Diversions and Digressions by Alan J. Rocke 4 Who first proposed the modern structure for pyridine? Old Chemistries by William D. Williams 6 Some literary mysteries for connoisseurs of old chemistry texts The History of the Dexter Award by Aaron I hde 11 Part IT of this continuing series explores the award's ftrst decade Bones and Stones by Fathi Habashi 14 The 250th anniversary of Canada's oldest ironworks Whatever Happened to ... ? by William Jensen 16 Before the atomic mass unit there was the microcrith - at least in American high school chemistry texts Chemical Artifacts by Leonard Fine 19 The rise and (literal) fall of the Chandler Museum at Columbia The Cover... Book Notes 22 Essays in Chemical History and Chemistry in America TIlls issue shows a caricature of Charles Frederick Chandler done on his 65th Translations 22 birthday. Chandler was Professor of Can you unravel this 18th century recipe for a pyrophorus? Chemistry at the Columbia School of Mines from 1864 to 1911 and thefoun Divisional News der of a unique chemical museum Report of the Program Chair 23 featured in this issue's CHEMICAL Awards 24 ARTIFACTS column. -

Final Exam Questions Generated by the Class
Final Exam Questions Generated by the Class Module 8 – Iron and Steel Describe some of the business practices that Carnegie employed that allowed him to take command of the steel industry. Hard driving, vertical integration, price making Which of the following was/is NOT a method used to make steel? A. Puddling B. Bessemer process C. Basic oxygen process D. Arc melting E. None of the above What are the three forms of iron, and what is the associated carbon content of each? Wrought <.2% Steel .2-2.3% Cast Iron 2.3-4.2% How did Andrew Carnegie use vertical integration to gain control of the steel market? Controlled the entire steel making process from mining to final product Who created the best steel for several hundred years while making swords during the 1500’s? A. Syria B. Egypt C. Japan D. England Describe the difference between forging and casting. When forging, you beat and hammer the material into the desired shape. When casting, you pour liquid into a mold to shape it. Describe the difference between steel and wrought iron. Steel has less carbon Which of the following forms of iron has a low melting point and is not forgeable? A. Steel B. Pig Iron C. Wrought Iron D. None of the Above What two developments ushered in the transition from the Bronze Age to the Iron Age? More iron ore and greater ability to change its properties using readily available alloying agent (carbon) 1 Final Exam Questions Generated by the Class What is the difference between ferrite and austenite? A. -

The Rate and Direction of Inventive Activity Revisited
This PDF is a selection from a published volume from the National Bureau of Economic Research Volume Title: The Rate and Direction of Inventive Activity Revisited Volume Author/Editor: Josh Lerner and Scott Stern, editors Volume Publisher: University of Chicago Press Volume ISBN: 0-226-47303-1; 978-0-226-47303-1 (cloth) Volume URL: http://www.nber.org/books/lern11-1 Conference Date: September 30 - October 2, 2010 Publication Date: March 2012 Chapter Title: The Rate and Direction of Invention in the British Industrial Revolution: Incentives and Institutions Chapter Authors: Ralf R. Meisenzahl, Joel Mokyr Chapter URL: http://www.nber.org/chapters/c12364 Chapter pages in book: (p. 443 - 479) 9 The Rate and Direction of Invention in the British Industrial Revolution Incentives and Institutions Ralf R. Meisenzahl and Joel Mokyr 9.1 Introduction The Industrial Revolution was the fi rst period in which technological progress and innovation became major factors in economic growth. There is by now general agreement that during the seventy years or so traditionally associated with the Industrial Revolution, there was little economic growth as traditionally measured in Britain, but that in large part this was to be expected.1 The sectors in which technological progress occurred grew at a rapid rate, but they were small in 1760, and thus their effect on growth was limited at fi rst (Mokyr 1998, 12– 14). Yet progress took place in a wide range of industries and activities, not just in cotton and steam. A full description of the range of activities in which innovation took place or was at least attempted cannot be provided here, but inventions in some pivotal industries such as iron and mechanical engineering had backward linkages in many more traditional industries. -
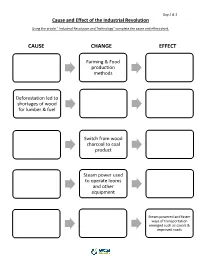
Cause Change Effect
Day 2 & 3 Cause and Effect of the Industrial Revolution Using the article “ Industrial Revolution and Technology” complete the cause and effect chart. CAUSE CHANGE EFFECT Farming & Food production methods Deforestation led to shortages of wood for lumber & fuel Switch from wood charcoal to coal product Steam power used to operate looms and other equipment Steam powered and faster ways of transportation emerged such as canals & improved roads Day 3 & 4 Industrial Revolution Timeline 1721: Cromford Mill was the first factory with the first water powered cotton spinning mill. 1733: Flying Shuttle made weaving faster, invented by John Kay. 1760: Industrial Revolution begins in England 1767: Spinning Jenny, a machine that can spin thread more quickly was invented James 1769: Water Frame, improved the Spinning Hargreaves. Jenny, ran on water instead of human labor. 1783: Henry Cort patented the puddling process which allowed wrought iron to be produced in large quantities. 1784: Steam Powered Loom that turned thread into cloth is invented by Edmund Cartwright. 1792-3: Eli Whitney invents the cotton gin, changing the textile sector. Now cotton fiber was separated from seeds through machine. 1831: Electric Generator led to improvements to create the first generator to support a factory. Invented by Michael Faraday. 1837: Telegraph made transmission of messages easier. Invented by William Fothergill & Charles Wheatstone. 1856: Bessemer Converter was the first process of manufacturing steel that wasn’t expensive. Day 3 & 4 Sources Brodsky Schur, J. (2016, September 23). Eli Whitney's Patent for the Cotton Gin. Retrieved June 22, 2021, from http://www.archives.gov/education/lessons/cotton-gin-patent Karpiel, F., Krull, K., & Wiggins, G.