Cognitive Behavior Therapy for Insomnia in Those with Depression
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Flowers for Algernon.Pdf
SHORT STORY FFlowerslowers fforor AAlgernonlgernon by Daniel Keyes When is knowledge power? When is ignorance bliss? QuickWrite Why might a person hesitate to tell a friend something upsetting? Write down your thoughts. 52 Unit 1 • Collection 1 SKILLS FOCUS Literary Skills Understand subplots and Reader/Writer parallel episodes. Reading Skills Track story events. Notebook Use your RWN to complete the activities for this selection. Vocabulary Subplots and Parallel Episodes A long short story, like the misled (mihs LEHD) v.: fooled; led to believe one that follows, sometimes has a complex plot, a plot that con- something wrong. Joe and Frank misled sists of intertwined stories. A complex plot may include Charlie into believing they were his friends. • subplots—less important plots that are part of the larger story regression (rih GREHSH uhn) n.: return to an earlier or less advanced condition. • parallel episodes—deliberately repeated plot events After its regression, the mouse could no As you read “Flowers for Algernon,” watch for new settings, charac- longer fi nd its way through a maze. ters, or confl icts that are introduced into the story. These may sig- obscure (uhb SKYOOR) v.: hide. He wanted nal that a subplot is beginning. To identify parallel episodes, take to obscure the fact that he was losing his note of similar situations or events that occur in the story. intelligence. Literary Perspectives Apply the literary perspective described deterioration (dih tihr ee uh RAY shuhn) on page 55 as you read this story. n. used as an adj: worsening; declining. Charlie could predict mental deterioration syndromes by using his formula. -

Book Club Kits by Author and Title Updated 3/2019
Book Club Kits by author and title Updated 3/2019 Fiction by author Gruen, Sara. Water for Elephants Rogan, Charlotte. Lifeboat Adams, Douglas. Hitchhiker’s Guide to Guinn, Jeff. Autobiography of Santa Scottoline, Lisa. Come Home the Galaxy Claus Scottoline, Lisa. Save Me Albom, Mitch. Time Keeper Hannah, Kristin. Firefly Lane Shaffer, Mary Ann. Guernsey Alcott, Louisa May. Little Women Hannah, Kristin. Great Alone Literary and Potato Peel Pie Allen, Sarah Addison. Peach Keeper Hannah, Kristin. Home Front Society Backman, Fredrik. A Man Called Ove Hannah, Kristin. Night Road Shattuck, Jessica. The Women in Baker, Ellen. I Gave My Heart to Know Hannah, Kristin. Nightingale the Castle This Harbach, Chad. Art of Fielding Shreve, Anita. Rescue Baldacci, David. Innocent Hawkins, Paula. Girl on the Train Simonson, Helen. Major Barclay, Linwood. Trust Your Eyes Hendricks, Greer and Sarah Pettigrew’s Last Stand Benjamin, Melanie. Aviator’s Wife Pekkanen. Wife Between Us Sittenfeld, Curtis. Sisterland Binchy, Maeve. Week in Winter Hilderbrand, Elin. Silver Girl Smith, Wilbur. Desert God Bohjalian, Chris. Sandcastle Girls Hilderbrand, Elin. Summerland Sparks, Nicholas. Safe Haven Brown, Eleanor. Weird Sisters Hosseini, Khaled. And the Stedman, M. L. Light between Campbell, Bonnie Jo. Once upon a Mountains Echoed Oceans River Jones, Tayari. An American Stockett, Kathryn. The Help Castillo, Linda. Her Last Breath Marriage Stoker, Bram. Dracula Chiaverini, Jennifer. Mrs. Lincoln’s King, Stephen. 11/22/63 Toibin, Colm. Brooklyn Dressmaker King, Stephen. Doctor Sleep Towles, Amor. Gentleman in Cline, Ernest. Ready Player One Kingsolver, Barbara. Flight Behavior Moscow Coben, Harlan. Six Years Landay, William. Defending Jacob VanLiere, Donna. Good Dream Daly, Maureen. -

Sleep Toolkit
Toolkit Sleep This tool kit works alongside the associated Parent Voice Factsheet. The resources will allow you to put some of the hints and tips into action and can be personalised to suit your child, family or circumstances. In this toolkit you will find the following resources— Sleep Factsheet A visual schedule template for you to personalise to fit your child’s bedtime routine A sleep diary template An environmental / personal observations template A reward chart template which could be adapted to your child’s interests A personal sleep story book that you can personalise to best support your child Sleep related clip art A list of further reading suggestions Sleep related vocabulary to use with your child A list of sleep related resources that other parents have found helpful www.hampshiregateway.info www.parentvoice.info Further reading Sleep Books This book list contains titles that can be used with children or to support families when they are developing sleep routines. What to Do When You Dread Your Bed: A Kid's Guide to Overcoming Problems with Sleep by Dawn Huebner (Author), Bon- nie Matthews (Contributor) Wouldn't it be great if you could climb into bed, snuggle under your covers, and fall asleep without any fuss or fear? Without listening for noises or thinking about bad guys? Without an extra drink, or an extra hug, or an extra trip to the bathroom? Bedtime is tough for many kids. If you're a kid who dreads your bed, and are convinced that nothing short of magic will make night time easier, this book is for you. -

Caecilia V60n08 1933
'lj. .35 .60 .80 .40 .60 .25 ... ~ . Tpe .Bruce PublishIng Cp., 524-544 ~. M.ilwaukeeSt;;,KliIw~~ke~, W~. Editor OTTO A. SINGENBERGER Professor of Gregorian Chant St. Mary of the Lake Seminary Mundelein, tllinois Manager WM. ARTHUR REILLY Chairman Boston School Committee Con~ributors LUDWIG BONVIN S.J. Buffalo, N. Y. GREGORY HUGLE, O.S.B. Conception Abbey Conception, Mo. JUSTIN FIELD, O.P. Diocesan Director of Church Music Alexandria, Onto SR. M. CHERUBIM O.S.F. Milwaukee, Wise. SR. M. GISELA, S.S.N.D. Milwaukee, Wisc. Founded A.D. 1874 by JOHN SINGENBERGER REMY ZADRA, D.D. Published by McLAUGHLIN & REILLY CO., 100 Boylston St., Boston, Mass. Jamestown, N. Y. M. MAURO-COTTONE Entered as second class matter. Oct. 20, 1931, at the post office at Boston, New York City Mass., under the act of March 3. 1879. Published monthly, except in July. Terms: Subscription price $2.00 per year. Canada and foreign countries PAUL C. TONNER Collegeville, Ind. $3.00 Payable in advance. Single copies 30 cents. Vol. 60 AUGUST 1933 No.8 irbiratinu TO 'THE VERY REVEREND GR.EGORY HUGLE O.S.B. Prior of Conception Abbey, Missouri. Contributor to the Church Music journals of America and many foreign countries, during the pa'stfifty years. Authority on Gregorian Chant. Humble and Beloved Priest. This issue is Respectfully Dedicated. 226 The Caecilia EDITORIAL Two years ago we began the policy of ded BIOGRAPHY icating the summer issue of CAECILIA to DOM GREGORY HU·GLE O.S.B. some living ...J\merican Catholic Church Mu sician of note, whose deeds and contributions Dom Gregory Hiigle was born in Lellwanr had won wide recognition in church music gen, in the archduchy of Baden, Germany, circles throughout this country and foreign September la, 1866. -

Satisfaction Rolling Stones 1965 3 American Pie Don Mclean 1972 4
AS VOTED AT OLDIESBOARD.COM 10/30/17 THROUGH 12/4/17 CONGRATULATIONS TO “HEY JUDE”, THE #1 SELECTION FOR THE 19 TH TIME IN 20 YEARS! Ti tle Artist Year 1 Hey Jude Beatles 1968 2 (I Can’t Get No) Satisfaction Rolling Stones 1965 3 American Pie Don McLean 1972 4 Light My Fire Doors 1967 5 In The Still Of The Nite Five Satins 1956 6 I Want To Hold Your Hand Beatles 1964 7 MacArthur Park Richard Harris 1968 8 Rag Doll Four Seasons 1964 9 God Only Knows Beach Boys 1966 10 Ain't No Mount ain High Enough Diana Ross 1970 11 Bridge Over Troubled Water Simon and Garfunkel 1970 12 Because Dave Clark Five 1964 13 Good Vibrations Beach Boys 1966 14 Cherish Association 1966 15 She Loves You Beatles 1964 16 Hotel California Eagles 1977 17 St airway To Heaven Led Zeppelin 1971 18 Born To Run Bruce Springsteen 1975 19 My Girl Temptations 1965 20 Let It Be Beatles 1970 21 Be My Baby Ronettes 1963 22 Downtown Petula Clark 1965 23 Since I Don't Have You Skyliners 1959 24 To Sir With Love Lul u 1967 25 Brandy (You're A Fine Girl) Looking Glass 1972 26 Suspicious Minds Elvis Presley 1969 27 You've Lost That Lovin' Feelin' Righteous Brothers 1965 28 You Really Got Me Kinks 19 64 29 Wichita Lineman Glen Campbell 1968 30 The Rain The Park & Ot her Things Cowsills 1967 31 A Hard Day's Night Beatles 1964 32 A Day In The Life Beatles 1967 33 Rock Around The Clock Bill Haley & His Comets 1955 34 Imagine John Lennon 1971 35 I Only Have Eyes For You Flamingos 1959 36 Waterloo Sunset Kinks 1967 37 Bohemian Rhapsody Queen 76 -92 38 Sugar Sugar Archies 1969 39 What's -

Escaping Eden
Escaping Eden Chapter 1 I wake up screaming. Where am I? What’s going on? Why do I feel so cold? It’s dark. I look around, blinking sleep from my eyes. I notice a window somewhere in the back of the room. It’s open and moonlight shines through, casting a square beam of light across the floor. A breeze drifts in, and white curtains float like pale ghosts in the darkness. In the narrow light of the moon, I see that the carpet is burgundy. I also see the outlines of furniture. My head vaguely aching, I look at the shadows scattered messily across the room. I feel like sweeping them up to make room for light. I shake off the urge. Stop being delusional. There’s a funny smell in the air, like a mixture of old home mustiness and laboratory sterilization. I realize that I’m lying on the floor. Why am I not on the bed? The last thing I remember is pain. Maybe that’s why I woke up screaming. The pain was intense. The pain was… cold. I decide to swallow my fear. It tastes terrible. I stand up and my neck aches. As I rub my hand across it, my bones crack like I haven’t moved in a long time. I whisper to myself. “As Grandpa always said…” My train of thought rolls off into the distance. Where was I going with this? Who is “Grandpa”? I put my hand on my sweaty forehead. I must be tired. I look for a light switch by approaching the walls and groping around. -

FWWH Revised Songbook ―This Camp Was Built to Music Therefore Built Forever
FWWH Revised Songbook Revised Summer 2011 ―This camp was built to music therefore built forever‖ These are the songs sung by Four Winds and Westward Ho campers – songs that have expressed their interests and ideals through the years. As you sing the songs again, may they recall memories of sunny days, and some misty and rainy ones too, of sailing on sparkling blue water, of cantering along leafy trails, of exploring the beach when the tide is out. May these songs remind you of unexpected adventure, and of friendships formed through the sharing of Summer days, working and playing together. 1 Index of songs A Gypsy‘s Life…………………………………………………….7 A Junior Song……………………………………………………..7 A Walking Song………………………………….…….………….8 Across A Thousand Miles of Sea…………..………..…………….8 Ah, Lovely Meadows…………………………..……..…………...9 All Hands On Deck……………………………………..……..…10 Another Fall…………………………………...…………………10 The Banks of the Sacramento…………………………………….…….12 Big Foot………………………………………..……….………………13 Bike Song……………………………………………………….…..…..14 Blow the Man Down…………………………………………….……...14 Blowin‘ In the Wind…………………………………………………....15 Boy‘s Grace…………………………………………………………….16 Boxcar……………………………………………………….…..……..16 Canoe Round…………………………………………………...………17 Calling Out To You…………………………………………………….17 Canoe Song……………………………………………………………..18 Canoeing Song………………………………………………………….18 Cape Anne………………………………………………...……………19 Carlyn…………………………………………………………….…….20 Changes………………………………………………………………...20 Christmas Night………………………………………………………...21 Christmas Song…………………………………………………………21 The Circle Game……………………………………………………..…22 -

Yamaha – Pianosoft Solo 29
YAMAHA – PIANOSOFT SOLO 29 SOLO COLLECTIONS ARTIST SERIES JOHN ARPIN – SARA DAVIS “A TIME FOR LOVE” BUECHNER – MY PHILIP AABERG – 1. A Time for Love 2. My Foolish FAVORITE ENCORES “MONTANA HALF LIGHT” Heart 3. As Time Goes By 4.The 1. Jesu, Joy Of Man’s Desiring 1. Going to the Sun 2. Montana Half Light 3. Slow Dance More I See You 5. Georgia On 2. “Bach Goes to Town” 4.Theme for Naomi 5. Marias River Breakdown 6.The Big My Mind 6. Embraceable You 3. Chanson 4. Golliwog’s Cake Open 7. Madame Sosthene from Belizaire the Cajun 8. 7. Sophisticated Lady 8. I Got It Walk 5. Contradance Diva 9. Before Barbed Wire 10. Upright 11. The Gift Bad and That Ain’t Good 9. 6. La Fille Aux Cheveux De Lin 12. Out of the Frame 13. Swoop Make Believe 10.An Affair to Remember (Our Love Affair) 7. A Giddy Girl 8. La Danse Des Demoiselles 00501169 .................................................................................$34.95 11. Somewhere Along the Way 12. All the Things You Are 9. Serenade Op. 29 10. Melodie Op. 8 No. 3 11. Let’s Call 13.Watch What Happens 14. Unchained Melody the Whole Thing Off A STEVE ALLEN 00501194 .................................................................................$34.95 00501230 .................................................................................$34.95 INTERLUDE 1. The Song Is You 2. These DAVID BENOIT – “SEATTLE MORNING” SARA DAVIS Foolish Things (Remind Me of 1. Waiting for Spring 2. Kei’s Song 3. Strange Meadowlard BUECHNER PLAYS You) 3. Lover Man (Oh Where 4. Linus and Lucy 5. Waltz for Debbie 6. Blue Rondo a la BRAHMS Can You Be) 4. -

Sleep Quality Study
SLEEP QUALITY STUDY Field November 16-17, 2020 N=183 completes This research is not just another COVID-19 survey. This study was conducted using immersive mobile messaging-based conversational exercises that capture robust quant data and emotive qual inputs in real-time from our mobile COVID-19 community members in one seamless experience. ● Most people are currently feeling stressed/worried/anxious or relaxed, in general. ● Most believe that they get 6-8 hours of sleep per night. A handful mention getting less than 5 hours of sleep, and a few mentio n more than 9 hours, but this is less common. ● Just over half (57%) say that they are getting the same amount of sleep now compared to before COVID ● Interestingly, almost 1/3 claim to be getting less sleep now than before COVID. o LESS SLEEP: Stress is the top reason why people think they are getting less sleep now than before COVID. People have too much on their minds, and it is affecting the amount and quality of sleep that they are getting. Source of stress range from personal issues (upcoming surgery, pregnancy, politics, work, loneliness, school schedule etc.) “Right now, my husband is due with knee surgery #4 so there's a lot to take care of. Especially the house. We needed to fly in help for the kids so to get everything ready and a little stocked up too because it'll be snowing… has kept me up at all hours.” “I really wish I knew. Stress about not being as productive, perhaps, or the feeling of being sequestered and not being able to travel or do many things that I enjoy. -

Getting Your Baby to Sleep the Baby Sleep Trainer Way
Copyright © 2017 by Natalie Willes. All Rights Reserved. Printed in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. ISBN: 978-0-9990867-0-4 Illustrator: Graphic design by Eliza Frye For Olive and Milo: Without you there would be none of this. CONTENTS Introduction ..................................v How To Use This Book ..........................1 The Science Of Sleep ...........................7 Bedroom Environment .........................13 The Elephant In The Room: Crying ...............19 Newborn Sleep ...............................27 Nighttime Sleep Training .......................43 Nap Training ................................81 Sleep Training Children In Beds .................105 Congrats! ..................................119 INTRODUCTION If you’re reading this book, you likely fall into one of two categories: You’re either a type-A, get-‘er-done parent who is trying to preempt any sleep problems by tackling them early or preventing them before they start, or you are the parent of an infant or toddler, and completely and utterly exhausted because your child is struggling to sleep well. Either way, this book is for you! I’d like to first address the latter group. I’ve spent the better part of the last five years working with hundreds of families across the world to solve their children’s sleep issues. Parents come to me having read multiple books, spoken to dozens of friends, and scoured the entire Internet trying to figure just how to help their beautiful and precious child sleep through the night and take healthful, restorative naps during the day. -

1 Giant Leap Dreadlock Holiday -- 10Cc I'm Not in Love
Dumb -- 411 Chocolate -- 1975 My Culture -- 1 Giant Leap Dreadlock Holiday -- 10cc I'm Not In Love -- 10cc Simon Says -- 1910 Fruitgum Company The Sound -- 1975 Wiggle It -- 2 In A Room California Love -- 2 Pac feat. Dr Dre Ghetto Gospel -- 2 Pac feat. Elton John So Confused -- 2 Play feat. Raghav & Jucxi It Can't Be Right -- 2 Play feat. Raghav & Naila Boss Get Ready For This -- 2 Unlimited Here I Go -- 2 Unlimited Let The Beat Control Your Body -- 2 Unlimited Maximum Overdrive -- 2 Unlimited No Limit -- 2 Unlimited The Real Thing -- 2 Unlimited Tribal Dance -- 2 Unlimited Twilight Zone -- 2 Unlimited Short Short Man -- 20 Fingers feat. Gillette I Want The World -- 2Wo Third3 Baby Cakes -- 3 Of A Kind Don't Trust Me -- 3Oh!3 Starstrukk -- 3Oh!3 ft Katy Perry Take It Easy -- 3SL Touch Me, Tease Me -- 3SL feat. Est'elle 24/7 -- 3T What's Up? -- 4 Non Blondes Take Me Away Into The Night -- 4 Strings Dumb -- 411 On My Knees -- 411 feat. Ghostface Killah The 900 Number -- 45 King Don't You Love Me -- 49ers Amnesia -- 5 Seconds Of Summer Don't Stop -- 5 Seconds Of Summer She Looks So Perfect -- 5 Seconds Of Summer She's Kinda Hot -- 5 Seconds Of Summer Stay Out Of My Life -- 5 Star System Addict -- 5 Star In Da Club -- 50 Cent 21 Questions -- 50 Cent feat. Nate Dogg I'm On Fire -- 5000 Volts In Yer Face -- 808 State A Little Bit More -- 911 Don't Make Me Wait -- 911 More Than A Woman -- 911 Party People.. -
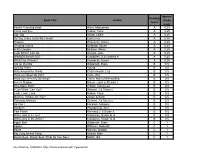
Book Title Author Reading Level Approx. Grade Level
Approx. Reading Book Title Author Grade Level Level Anno's Counting Book Anno, Mitsumasa A 0.25 Count and See Hoban, Tana A 0.25 Dig, Dig Wood, Leslie A 0.25 Do You Want To Be My Friend? Carle, Eric A 0.25 Flowers Hoenecke, Karen A 0.25 Growing Colors McMillan, Bruce A 0.25 In My Garden McLean, Moria A 0.25 Look What I Can Do Aruego, Jose A 0.25 What Do Insects Do? Canizares, S.& Chanko,P A 0.25 What Has Wheels? Hoenecke, Karen A 0.25 Cat on the Mat Wildsmith, Brain B 0.5 Getting There Young B 0.5 Hats Around the World Charlesworth, Liza B 0.5 Have you Seen My Cat? Carle, Eric B 0.5 Have you seen my Duckling? Tafuri, Nancy/Greenwillow B 0.5 Here's Skipper Salem, Llynn & Stewart,J B 0.5 How Many Fish? Cohen, Caron Lee B 0.5 I Can Write, Can You? Stewart, J & Salem,L B 0.5 Look, Look, Look Hoban, Tana B 0.5 Mommy, Where are You? Ziefert & Boon B 0.5 Runaway Monkey Stewart, J & Salem,L B 0.5 So Can I Facklam, Margery B 0.5 Sunburn Prokopchak, Ann B 0.5 Two Points Kennedy,J. & Eaton,A B 0.5 Who Lives in a Tree? Canizares, Susan et al B 0.5 Who Lives in the Arctic? Canizares, Susan et al B 0.5 Apple Bird Wildsmith, Brain C 1 Apples Williams, Deborah C 1 Bears Kalman, Bobbie C 1 Big Long Animal Song Artwell, Mike C 1 Brown Bear, Brown Bear What Do You See? Martin, Bill C 1 Found online, 7/20/2012, http://home.comcast.net/~ngiansante/ Approx.