Strategy for the Establishment of Self-Sustaining Atlantic Whitefish Population(S) and Development of a Framework for the Evaluation of Suitable Lake Habitat
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Burbot Management Plan
BURBOT MANAGEMENT PLAN DEPARTMENT OF INLAND FISHERIES AND WILDLIFE DIVISION OF FISHERIES AND HATCHERIES PREPARED BY SCOTT A. ROY ASSISTANT REGIONAL FISHERIES BIOLOGIST REGION E MARCH 2001 BURBOT LIFE HISTORY The burbot, Lota lota (Linnaeus), is a unique member of the cod family. It is the only species in the family, which spends its entire life in fresh water. However, the burbot is similar to its marine relatives in that its distribution is circumpolar. It can be found in cool, fresh waters throughout northern Europe, Asia and North America. In North America its range extends as far south as the northern tier of States across the United States. In Maine, the burbot is commonly known as the cusk, although in other areas it is also called the ling, eelpout, loche and lawyer. Unlike the salmonids and Maine’s other coldwater species, the burbot is not noted for its grace and beauty. The body is elongate, almost eel-shaped, with long, soft-rayed dorsal and anal fins that meet a rounded tail. Although it is smooth and slimy to the touch, the skin is embedded with very small, cycloid scales. The head of the burbot is broad and somewhat flattened. It has a large mouth containing several rows of small teeth on the jaws. A single, whisker-like barbell protrudes from the tip of the chin. There are no obvious external differences between males and females. In general, adults are olive brown to dark brown on the back and sides. This background is overlaid with distinctive patterns of dark brown or black markings and spots. -

Sport-Fish-Identification.Pdf
Walleye Walleye have two distinct fins on their back, the first with large spines. Lake Sturgeon They have a yellow-olive back, brassy, silvery sides with yellow spots, a white underside, and white on the lower lobe of the tail. Dusky vertical Lake Sturgeon are a Threatened Species due to population size and bars are often found on the body as well. concerns with viability. Lake Sturgeon have a large brown or grey body covered with tough, leather- like tissue and five rows of bony plates. They have a shark-like, upturned tail and a pointed snout with four barbels. Sauger Lake Whitefish are olive-green to blue on the back, with silvery sides.They Sauger are a Threatened Species due to hybridization, habitat Lakehave a small Whitefish mouth below a rounded snout, and a deeply forked tail. degradation and overharvest. Sauger are golden olive on the back with silver-yellow sides and a white underside. They also have a large spiny dorsal fin, distinct rows of spots on the dorsal fins and three or four dusky vertical bars on the body. Mountain Whitefish have large scales, no spots and small mouths with no Burbot Mountainteeth. Their general Whitefish body colour is a bronze-white or greenish white. Burbot have a slim, brownish black body with smooth skin, a flattened head, and a fin that stretches along the back half of the body. Distinctive barbels hang from the lower jaw and nostrils. Goldeye Northern Pike Goldeye have prominent eyes with bright yellow pupils, a blunt head, and Northern Pike are a long, slender fish with duck-like jaws and a long, flat a deep, compressed body. -

Westslope Cutthroat Trout
This file was created by scanning the printed publication. Errors identified by the software have been corrected; Chapter 1 however, some errors may remain. Westslope Cutthroat Trout John D. IVIclntyre and Bruce E. Rieman, USDA Forest Service, Intermountain Research Station, 316 E. iViyrtle Street, Boise, Idaho 83702 Introduction Westslope cutthroat trout begin to mature at age 3 but usually spawn first at age 4 or 5 (table 2). Sexu The westslope cutthroat trout inhabits streams on ally maturing adfluvial fish move into the vicinity of both sides of the Continental Divide. On the east side tributaries in fall and winter where they remain un of the divide, they are distributed mostly in Mon til they begin to migrate upstream in the spring tana but also occur in some headwaters in Wyoming (Liknes 1984). They spawn from March to July at and southern Alberta (Behnke 1992). They are in the water temperatures near 10°C (Roscoe 1974; Liknes Missouri Basin downstream to about 60 km below 1984; Shepard et al. 1984). A population of adult fish Great Falls and in the headwaters of the Judith, Milk, in the St. Joe River, Idaho, included 1.6 females for and Marias rivers. On the west side of the Continen each male (Thurow and Bjornn 1978). Average length tal Divide the subspecies occurs in the upper was 334 mm for females and 366 mm for males. A Kootenai River; the Clark Fork drainage in Montana similar population in Big Creek, Montana, included and Idaho downstream to the falls on the Fend Oreille 4.1 females for each male (Huston et al. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Traceability Study in Shark Products
Traceability study in shark products Dr Heiner Lehr (Photo: © Francisco Blaha, 2015) Report commissioned by the CITES Secretariat This publication was funded by the European Union, through the CITES capacity-building project on aquatic species Contents 1 Summary.................................................................................................................................. 7 1.1 Structure of the remaining document ............................................................................. 9 1.2 Acknowledgements ....................................................................................................... 10 2 The market chain ................................................................................................................... 11 2.1 Shark Products ............................................................................................................... 11 2.1.1 Shark fins ............................................................................................................... 12 2.1.2 Shark meat ............................................................................................................. 12 2.1.3 Shark liver oil ......................................................................................................... 13 2.1.4 Shark cartilage ....................................................................................................... 13 2.1.5 Shark skin .............................................................................................................. -

Lake Superior Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake Superior Food Web MENT OF C Sea Lamprey Walleye Burbot Lake Trout Chinook Salmon Brook Trout Rainbow Trout Lake Whitefish Bloater Yellow Perch Lake herring Rainbow Smelt Deepwater Sculpin Kiyi Ruffe Lake Sturgeon Mayfly nymphs Opossum Shrimp Raptorial waterflea Mollusks Amphipods Invasive waterflea Chironomids Zebra/Quagga mussels Native waterflea Calanoids Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake Superior, 2009. 734-741-2235 - www.glerl.noaa.gov Lake Superior Food Web Sea Lamprey Macroinvertebrates Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Chironomids/Oligochaetes. Larval insects and worms that live on the lake fastens onto its prey and rasps out a hole with its rough tongue. bottom. Feed on detritus. Species present are a good indicator of water quality. Piscivores (Fish Eaters) Amphipods (Diporeia). The most common species of amphipod found in fish diets that began declining in the late 1990’s. Chinook salmon (Oncorhynchus tshawytscha). Pacific salmon species stocked as a trophy fish and to control alewife. Opossum shrimp (Mysis relicta). An omnivore that feeds on algae and small cladocerans. -

Differences in Brain Transcriptomes of Closely Related Baikal Coregonid Species
Differences in brain transcriptomes of closely related baikal coregonid species Bychenko, O. S., Sukhanova, L. V., Azhikina, T. L., Skvortsov, T. A., Belomestnykh, T. V., & Sverdlov, E. D. (2014). Differences in brain transcriptomes of closely related baikal coregonid species. BioMed Research International, 2014, [857329]. DOI: 10.1155/2014/857329 Published in: BioMed Research International Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright 2017 the authors. This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:06. Nov. 2017 Hindawi Publishing Corporation BioMed Research International Volume 2014, Article ID 857329, 11 pages http://dx.doi.org/10.1155/2014/857329 Research Article Differences in Brain Transcriptomes of Closely Related Baikal Coregonid Species Oksana S. -

Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe
Identification and Modelling of a Representative Vulnerable Fish Species for Pesticide Risk Assessment in Europe Von der Fakultät für Mathematik, Informatik und Naturwissenschaften der RWTH Aachen University zur Erlangung des akademischen Grades eines Doktors der Naturwissenschaften genehmigte Dissertation vorgelegt von Lara Ibrahim, M.Sc. aus Mazeraat Assaf, Libanon Berichter: Universitätsprofessor Dr. Andreas Schäffer Prof. Dr. Christoph Schäfers Tag der mündlichen Prüfung: 30. Juli 2015 Diese Dissertation ist auf den Internetseiten der Universitätsbibliothek online verfügbar Erklärung Ich versichere, dass ich diese Doktorarbeit selbständig und nur unter Verwendung der angegebenen Hilfsmittel angefertigt habe. Weiterhin versichere ich, die aus benutzten Quellen wörtlich oder inhaltlich entnommenen Stellen als solche kenntlich gemacht zu haben. Lara Ibrahim Aachen, am 18 März 2015 Zusammenfassung Die Zulassung von Pflanzenschutzmitteln in der Europäischen Gemeinschaft verlangt unter anderem eine Abschätzung des Risikos für Organismen in der Umwelt, die nicht Ziel der Anwendung sind. Unvertretbare Auswirkungen auf den Naturhalt sollen vermieden werden. Die ökologische Risikoanalyse stellt die dafür benötigten Informationen durch eine Abschätzung der Exposition der Organismen und der sich daraus ergebenden Effekte bereit. Die Effektabschätzung beruht dabei hauptsächlich auf standardisierten ökotoxikologischen Tests im Labor mit wenigen, oft nicht einheimischen Stellvertreterarten. In diesen Tests werden z. B. Effekte auf das Überleben, das Wachstum und/oder die Reproduktion von Fischen bei verschiedenen Konzentrationen der Testsubstanz gemessen und Endpunkte wie die LC50 (Lethal Concentrations for 50%) oder eine NOEC (No Observed Effect Concentration, z. B. für Wachstum oder Reproduktionsparameter) abgeleitet. Für Fische und Wirbeltiere im Allgemeinen beziehen sich die spezifischen Schutzziele auf das Überleben von Individuen und die Abundanz und Biomasse von Populationen. -
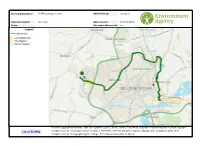
GIS Screening
Screening Results for: IR EPR discharges to water Date Produced: 16/09/2020 Reference number: KP3236YH NGR of search: SP 86093 68828 Notes: Site centre distance (m): 80 https://easimap2. prodds. ntnl/Html5Viewer/I ndex.html? viewer=Permitting &Extent=484159. 913849983, 266371. 936523897, © Crown Copyright and database right 2020. Ordnance Survey licence number 100024198. Copyright © Natural England. © Crown Copyright. 492626. Launch597450017, EasiMap All rights reserved. Countryside Council for Wales, 100018813, 2020. © Environment Agency copyright and / or database rights 2020. 270889. All rights reserved. © Copyright English Heritage. © The National Assembly for Wales. Easimap Screening Results Click here to download Permit Screening distances Screening Results for: IR EPR discharges to water Date produced: 16/09/2020 Reference number: KP3236YH NGR of search: SP 86093 68828 Notes: Site centre distance (m): 80 Data Details Within Search Direction Action Special Protection Areas - England Site Name: Upper Nene Valley Gravel Pits 9813m Downstream in the HRA Stage 1 Form (App11) consultation with NE may be required (SPA) nearest watercourse Ramsar Sites - England Site Name: Upper Nene Valley Gravel Pits 9813m Downstream in the HRA Stage 1 Form (App11) consultation with NE may be required (Ramsar) nearest watercourse Local Wildlife Sites Name: Park Farm Industrial Estate 424m Downstream in the Take account of the requirements of this site. If there is no data available nearest watercourse consult Area EPR Installation Team EPR Team: Installations -

Ours to Save: the Distribution, Status & Conservation Needs of Canada's Endemic Species
Ours to Save The distribution, status & conservation needs of Canada’s endemic species June 4, 2020 Version 1.0 Ours to Save: The distribution, status & conservation needs of Canada’s endemic species Additional information and updates to the report can be found at the project website: natureconservancy.ca/ourstosave Suggested citation: Enns, Amie, Dan Kraus and Andrea Hebb. 2020. Ours to save: the distribution, status and conservation needs of Canada’s endemic species. NatureServe Canada and Nature Conservancy of Canada. Report prepared by Amie Enns (NatureServe Canada) and Dan Kraus (Nature Conservancy of Canada). Mapping and analysis by Andrea Hebb (Nature Conservancy of Canada). Cover photo credits (l-r): Wood Bison, canadianosprey, iNaturalist; Yukon Draba, Sean Blaney, iNaturalist; Salt Marsh Copper, Colin Jones, iNaturalist About NatureServe Canada A registered Canadian charity, NatureServe Canada and its network of Canadian Conservation Data Centres (CDCs) work together and with other government and non-government organizations to develop, manage, and distribute authoritative knowledge regarding Canada’s plants, animals, and ecosystems. NatureServe Canada and the Canadian CDCs are members of the international NatureServe Network, spanning over 80 CDCs in the Americas. NatureServe Canada is the Canadian affiliate of NatureServe, based in Arlington, Virginia, which provides scientific and technical support to the international network. About the Nature Conservancy of Canada The Nature Conservancy of Canada (NCC) works to protect our country’s most precious natural places. Proudly Canadian, we empower people to safeguard the lands and waters that sustain life. Since 1962, NCC and its partners have helped to protect 14 million hectares (35 million acres), coast to coast to coast. -

(Acipenser Brevirostrum) in Canada Shortnose Sturgeon
Proposed Species at Risk Act Management Plan Series Management Plan for the Shortnose Sturgeon (Acipenser brevirostrum) in Canada Shortnose Sturgeon 2015 Management Plan for the Shortnose Sturgeon [Proposed] 2015 Recommended citation: Draft Fisheries and Oceans Canada. 2015. Management Plan for the Shortnose Sturgeon (Acipenser brevirostrum) in Canada [Proposed]. Species at Risk Act Management Plan Series. Fisheries and Oceans Canada, Ottawa. v + 55 pp. Additional copies: For copies of the Management Plan, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the SAR Public Registry. Cover illustration: DFO (2007) Également disponible en français sous le titre « Plan de gestion pour l’esturgeon à museau court (Acipenser brevirostrum) au Canada» © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2015. All rights reserved. ISBN ISBN to be included Catalogue no. Catalogue no. to be included Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. Management Plan for the Shortnose Sturgeon [Proposed] 2015 Preface Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of Management Plans for listed species of Special Concern and are required to report on progress within five years. The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. The Minister of Fisheries and Oceans is the competent minister under SARA for the Shortnose Sturgeon and has prepared this Management Plan as per section 65 of SARA. -

The State of Lake Huron in 2010 Special Publication 13-01
THE STATE OF LAKE HURON IN 2010 SPECIAL PUBLICATION 13-01 The Great Lakes Fishery Commission was established by the Convention on Great Lakes Fisheries between Canada and the United States, which was ratified on October 11, 1955. It was organized in April 1956 and assumed its duties as set forth in the Convention on July 1, 1956. The Commission has two major responsibilities: first, develop coordinated programs of research in the Great Lakes, and, on the basis of the findings, recommend measures which will permit the maximum sustained productivity of stocks of fish of common concern; second, formulate and implement a program to eradicate or minimize sea lamprey populations in the Great Lakes. The Commission is also required to publish or authorize the publication of scientific or other information obtained in the performance of its duties. In fulfillment of this requirement the Commission publishes the Technical Report Series, intended for peer-reviewed scientific literature; Special Publications, designed primarily for dissemination of reports produced by working committees of the Commission; and other (non-serial) publications. Technical Reports are most suitable for either interdisciplinary review and synthesis papers of general interest to Great Lakes fisheries researchers, managers, and administrators, or more narrowly focused material with special relevance to a single but important aspect of the Commission's program. Special Publications, being working documents, may evolve with the findings of and charges to a particular committee. Both publications follow the style of the Canadian Journal of Fisheries and Aquatic Sciences. Sponsorship of Technical Reports or Special Publications does not necessarily imply that the findings or conclusions contained therein are endorsed by the Commission.