The State of Lake Huron in 2010 Special Publication 13-01
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Seasonal and Diel Movements and Habitat Use of Robust Redhorses in the Lower Savannah River. Georgia, and South Carolina
Transactions of the American FisheriesSociety 135:1145-1155, 2006 [Article] © Copyright by the American Fisheries Society 2006 DO: 10.1577/705-230.1 Seasonal and Diel Movements and Habitat Use of Robust Redhorses in the Lower Savannah River, Georgia and South Carolina TIMOTHY B. GRABOWSKI*I Department of Biological Sciences, Clemson University, Clemson, South Carolina,29634-0326, USA J. JEFFERY ISELY U.S. Geological Survey, South Carolina Cooperative Fish and Wildlife Research Unit, Clemson University, Clemson, South Carolina, 29634-0372, USA Abstract.-The robust redhorse Moxostonta robustum is a large riverine catostomid whose distribution is restricted to three Atlantic Slope drainages. Once presumed extinct, this species was rediscovered in 1991. Despite being the focus of conservation and recovery efforts, the robust redhorse's movements and habitat use are virtually unknown. We surgically implanted pulse-coded radio transmitters into 17 wild adults (460-690 mm total length) below the downstream-most dam on the Savannah River and into 2 fish above this dam. Individuals were located every 2 weeks from June 2002 to September 2003 and monthly thereafter to May 2005. Additionally, we located 5-10 individuals every 2 h over a 48-h period during each season. Study fish moved at least 24.7 ± 8.4 river kilometers (rkm; mean ± SE) per season. This movement was generally downstream except during spring. Some individuals moved downstream by as much as 195 rkm from their release sites. Seasonal migrations were correlated to seasonal changes in water temperature. Robust redhorses initiated spring upstream migrations when water temperature reached approximately 12'C. Our diel tracking suggests that robust redhorses occupy small reaches of river (- 1.0 rkm) and are mainly active diumally. -

Spatial Criteria Used in IUCN Assessment Overestimate Area of Occupancy for Freshwater Taxa
Spatial Criteria Used in IUCN Assessment Overestimate Area of Occupancy for Freshwater Taxa By Jun Cheng A thesis submitted in conformity with the requirements for the degree of Masters of Science Ecology and Evolutionary Biology University of Toronto © Copyright Jun Cheng 2013 Spatial Criteria Used in IUCN Assessment Overestimate Area of Occupancy for Freshwater Taxa Jun Cheng Masters of Science Ecology and Evolutionary Biology University of Toronto 2013 Abstract Area of Occupancy (AO) is a frequently used indicator to assess and inform designation of conservation status to wildlife species by the International Union for Conservation of Nature (IUCN). The applicability of the current grid-based AO measurement on freshwater organisms has been questioned due to the restricted dimensionality of freshwater habitats. I investigated the extent to which AO influenced conservation status for freshwater taxa at a national level in Canada. I then used distribution data of 20 imperiled freshwater fish species of southwestern Ontario to (1) demonstrate biases produced by grid-based AO and (2) develop a biologically relevant AO index. My results showed grid-based AOs were sensitive to spatial scale, grid cell positioning, and number of records, and were subject to inconsistent decision making. Use of the biologically relevant AO changed conservation status for four freshwater fish species and may have important implications on the subsequent conservation practices. ii Acknowledgments I would like to thank many people who have supported and helped me with the production of this Master’s thesis. First is to my supervisor, Dr. Donald Jackson, who was the person that inspired me to study aquatic ecology and conservation biology in the first place, despite my background in environmental toxicology. -

The Majestic “Notty” Moonlight Bay Cottages – North Bay 1958 Glengarry Cottages 1966
The Majestic “Notty” Moonlight Bay Cottages – North Bay 1958 Glengarry Cottages 1966 Topics for Today •The Notty •The Nottawasaga Steelheaders • Great Fishery, Concerns and Issues •What can we do The Notty ... Majestic Lady Survival of a Great Watershed • Gary Christie • B. Sc. Biology (York) • Training (Sales- Medical & Life Sciences Research Clinical, DNA) • President (Since 2001) Nottawasaga Steelheaders • Passions – Fishing, Wine & Good Friends and…putting a little back with some volunteer work Gary at Wasaga Beach 1957 Nottawasaga The name is derived from Huron First Nation words referring to the “outlet of the river” where Iroquois would attack the Hurons. 1600 km 3500 sq km MW The Notty…did you know? • Notty Basin - 3500 sq km • 3 counties and 18 municipalities • main branch is over 120 km in length • 11 major tributaries >>McIntyre Creek, Little Marl Creek, Marl Creek, Willow Creek, Mad River, Bear Creek, Pine River, Boyne River, Innisfil Creek, Sheldon Creek and the Upper Nottawasaga reaching as far as Orangeville. • many species of fish, including pike, bass, walleye, sturgeon, brown trout, brook trout, rainbow trout, crappie, salmon – 75 Species of Fish • Home of Int’l recognized (RAMSAR) Minesing Wetland • Wasaga Beach historically important –War of 1812. HMS Nancy sunk defending great lakes • Notty was key lumber river in 1800’s and proposed rail line to Toronto from Wasaga Beach • Notty basin formed by Pleistocene era glaciers 20, 000 years ago • 3 Geological Features - Niagara Escarpment - Rolling Moraines - Broad Simcoe -

Michigan Study No.: 230703 Project No.: F-80-R-5 Title
STUDY PERFORMANCE REPORT State: Michigan Project No.: F-80-R-5 Study No.: 230703 Title: Lakewide assessment of the contribution of natural recruitment to the chinook salmon population of Lake Huron. Period Covered: October 1, 2003 to September 30, 2004 Study Objective: (1) To estimate annual natural recruitment of chinook salmon to Lake Huron for the 2000 to 2003 year classes; (2) To determine contributions from natural reproduction to the spawning populations of selected tributaries to Lake Huron; (3) To refine recruitment modules of Lake Huron’s bioenergetics and catch-at-age models, which will, in turn, be used to prescribe stocking levels for Lake Huron. Summary: This was the third year of funding for this project. All chinook salmon stocked in lakes Huron and Michigan, except those stocked by Ontario, were marked using oxytetracycline administered in feed. All chinook salmon stocked in Ontario waters of Lake Huron were fin clipped. Quality control samples of vertebrae were received during May and June 2003 from Michigan, Illinois, Indiana, and Wisconsin hatcheries and the samples were checked for quality of the oxytetracycline mark. We used ultraviolet microscope equipment and imaging software to enhance reproducibility and specimen processing speed. Vertebrae images and biological data from the Chinook salmon sampled were electronically archived in a database developed cooperatively with Ontario Ministry of Natural Resources. These data were shared with other cooperating agencies on the Lake Huron Technical Committee. This year was the third year of field collections and creel clerks and coded-wire tag recovery personnel were trained in gathering vertebrae for the recruitment study. -

Genetic Research on Commercially Exploited Fish Species in Nordic Countries
Genetic research on commercially exploited fish species in Nordic countries Jens Olsson, Teija Aho, Ann-Britt Florin, Anssi Vainikka, Dorte Bekkevold, Johan Dannewitz, Kjetil Hindar, Marja-Liisa Kol- jonen, Linda Laikre, Eyðfinn Magnussen, and Snæbjörn Pálsson. TemaNord 2007:542 Genetic research on commercially exploited fish species in Nordic countries TemaNord 2007:542 © Nordic Council of Ministers, Copenhagen 2007 ISBN 978-92-893-1508-1 This publication can be ordered on www.norden.org/order. Other Nordic publications are available at www.norden.org/publications Nordic Council of Ministers Nordic Council Store Strandstræde 18 Store Strandstræde 18 DK-1255 Copenhagen K DK-1255 Copenhagen K Phone (+45) 3396 0200 Phone (+45) 3396 0400 Fax (+45) 3396 0202 Fax (+45) 3311 1870 www.norden.org Nordic cooperation Nordic cooperation is one of the world’s most extensive forms of regional collaboration, involving Denmark, Finland, Iceland, Norway, Sweden, and three autonomous areas: the Faroe Islands, Green- land, and Åland. Nordic cooperation has firm traditions in politics, the economy, and culture. It plays an important role in European and international collaboration, and aims at creating a strong Nordic community in a strong Europe. Nordic cooperation seeks to safeguard Nordic and regional interests and principles in the global community. Common Nordic values help the region solidify its position as one of the world’s most innovative and competitive. Content Preface............................................................................................................................... -

Bear Lake Whitefish Prosopium Abyssicola
Bear Lake Whitefish Prosopium abyssicola Actinopterygii — Salmoniformes — Salmonidae CONSERVATION STATUS / CLASSIFICATION Rangewide: Critically imperiled (G1) Statewide: Critically imperiled (S1) ESA: No status USFS: Region 1: No status; Region 4: No status BLM: Rangewide/Globally imperiled (Type 2) IDFG: Game fish BASIS FOR INCLUSION Endemic to Bear Lake. TAXONOMY The Bear Lake whitefish is 1 of 3 sympatric members of the genus Prosopium. No subspecies has been proposed. DISTRIBUTION AND ABUNDANCE This species is endemic to Bear Lake. POPULATION TREND Monitoring for >20 years indicates the population is stable (Nielson and Tolentino 2002). HABITAT AND ECOLOGY The Bear Lake whitefish typically occurs in the benthic zone at water depths greater than 40 m (130 ft). Spawning occurs in mid–February to mid–March in shallow, rocky areas. Ostracods comprise the majority of the diet, but other invertebrates found on the lake bottom may be consumed. ISSUES The lowering of lake levels due to natural events and anthropogenic actions could limit spawning and rearing habitat. Increasing human development around the lake could lead to lowering of water quality due to waste water discharges. Legal and illegal introductions of piscivorous fish could affect populations by increased predation rate. RECOMMENDED ACTIONS Continue programs that (1) monitor the population status and trend; (2) evaluate the relationship between water quality and level and fish populations; (3) stock sterile triploid lake trout; and (4) removal of illegally introduced non–native fish (e.g., walleye) in conjuction with adjacent states. Bear Lake Whitefish Prosopium abyssicola Ecological Section Species Range 10 August 2005 Fish information is from Idaho Fish and Wildlife 0 20 40 80 Kilometers Information System, Idaho Deptartment of Fish and Game and displayed at the 6th code hydrologic unit. -

Fish Study Cover 3
Putnam County Environmental Council ! !"#"$%&%#'("#)(*%+',-"'.,#(,/( '0%(1.+0(2,345"'.,#+(,/(6.57%-( 63-.#$+("#)('0%(!.))5%("#)(8,9%-( :;<5"9"0"(*.7%-=(15,-.)"=(>6?( ( *,@(*A(8%9.+(BBB=(!A?A=(2ACA6A( MANAGEMENT AND RESTORATION OF THE FISH POPULATIONS OF SILVER SPRINGS AND THE MIDDLE AND LOWER OCKLAWAHA RIVER, FLORIDA, USA A Special Report for The Putnam County Environmental Council Funded by a Grant from the Felburn Foundation By Roy R. “Robin” Lewis III, M.A., P.W.S. Certified Professional Wetland Scientist and Certified Senior Ecologist May 14, 2012 Cover photograph: Longnose Gar, Lepisosteus osseus, in Silver Springs, Underwater Photograph by Peter Butt, KARST Environmental ACKNOWLEDGEMENTS The author wishes to thank all those who reviewed and commented on the numerous drafts of this document, including Paul Nosca, Michael Woodward, Curtis Kruer and Sandy Kokernoot. All conclusions, however, remain the responsibility of the author. CITATION The suggested citation for this report is: LEWIS, RR. 2012. MANAGEMENT AND RESTORATION OF THE FISH POPULATIONS OF SILVER SPRINGS AND THE MIDDLE AND LOWER OCKLAWAHA RIVER, FLORIDA, USA. Putnam County Environmental Council, Interlachen, Florida. 27 p + append. Additional copies of this document can be downloaded from the PCEC website at www.pcecweb.org. i EXECUTIVE SUMMARY Sixty‐nine (69) species of native fish have been documented to have utilized Silver Springs, Silver River and the Upper, Middle and Lower Ocklawaha River for the period of record. Fifty‐nine of these are freshwater fish species and ten are native migratory species using marine, estuarine and freshwater habitats during their life history. These include striped bass, American eel, American shad, hickory shad, hogchoker, striped mullet, channel and white catfish, needlefish and southern flounder. -

Version 2020-04-20 Bear Lake Whitefish (Prosopium Abyssicola
Version 2020-04-20 Bear Lake Whitefish (Prosopium abyssicola) Species Status Statement. Distribution Bear Lake whitefish is one of four fish species naturally found only in Bear Lake, which straddles the Utah-Idaho border. This species has also never been transplanted elsewhere, and occurs nowhere else in the world (Sigler and Sigler 1987). Table 1. Utah counties currently occupied by this species. Bear Lake Whitefish RICH Abundance and Trends Prior to 1999, there was simply no reliable method for fishery biologists to differentiate Bear Lake whitefish from Bonneville whitefish at lengths less than approximately 10 inches outside of their respective spawning seasons (Tolentino and Thompson 2004). Therefore, the Utah Division of Wildlife Resources (UDWR) monitored both species combined as the “whitefish complex”. In 1999, Ward (2001) along with UDWR biologists (Tolentino and Thompson 2004) finally described a reliable method to distinguish the two whitefish species in Bear Lake. From 1999-2018 the UDWR has monitored gill net catch rates and composition of Bonneville and Bear Lake whitefish separately (Tolentino 2007). The population of Bear Lake whitefish has appeared to remain stable from 1999-2017, comprising an average of 26% of the whitefish species caught in survey nets each year. Statement of Habitat Needs and Threats to the Species. Habitat Needs Bear Lake whitefish spend a majority of their life near the bottom of the lake’s deep waters. For most of each year, they live at depths ranging from 130 to 200 feet (Thompson 2003, Tolentino 2007). However, during the months of February and March the adult fish move into rocky, somewhat shallower areas (20-100 feet) to spawn (Tolentino and Albrecht 2007). -

Coregonus Nigripinnis) in Northern Algonquin Provincial Park
HABITAT PREFERENCES AND FEEDING ECOLOGY OF BLACKFIN CISCO (COREGONUS NIGRIPINNIS) IN NORTHERN ALGONQUIN PROVINCIAL PARK A Thesis Submitted to the Committee on Graduate Studies in Partial Fulfillment of the Requirements for the Degree of Master of Science in the Faculty of Arts and Science Trent University Peterborough, Ontario, Canada © Copyright by Allan Henry Miller Bell 2017 Environmental and Life Sciences M.Sc. Graduate Program September 2017 ABSTRACT Depth Distribution and Feeding Structure Differentiation of Blackfin Cisco (Coregonus nigripinnis) In Northern Algonquin Provincial Park Allan Henry Miller Bell Blackfin Cisco (Coregonus nigripinnis), a deepwater cisco species once endemic to the Laurentian Great Lakes, was discovered in Algonquin Provincial Park in four lakes situated within a drainage outflow of glacial Lake Algonquin. Blackfin habitat preference was examined by analyzing which covariates best described their depth distribution using hurdle models in a multi-model approach. Although depth best described their distribution, the nearly isothermal hypolimnion in which Blackfin reside indicated a preference for cold-water habitat. Feeding structure differentiation separated Blackfin from other coregonines, with Blackfin possessing the most numerous (50-66) gill rakers, and, via allometric regression, the longest gill rakers and lower gill arches. Selection for feeding efficiency may be a result of Mysis diluviana affecting planktonic size structure in lakes containing Blackfin Cisco, an effect also discovered in Lake Whitefish (Coregonus clupeaformis). This thesis provides insight into the habitat preferences and feeding ecology of Blackfin and provides a basis for future study. Keywords: Blackfin Cisco, Lake Whitefish, coregonine, Mysis, habitat, feeding ecology, hurdle models, allometric regression, Algonquin Provincial Park ii ACKNOWLEDGEMENTS First and foremost I would like to thank my supervisor Dr. -

Food‐Web Structure and Ecosystem Function in the Laurentian Great
Received: 13 March 2018 | Revised: 14 September 2018 | Accepted: 18 September 2018 DOI: 10.1111/fwb.13203 REVIEW Food- web structure and ecosystem function in the Laurentian Great Lakes—Toward a conceptual model Jessica T. Ives1 | Bailey C. McMeans2 | Kevin S. McCann3 | Aaron T. Fisk4 | Timothy B. Johnson5 | David B. Bunnell6 | Kenneth T. Frank7 | Andrew M. Muir1 1Great Lakes Fishery Commission, Ann Arbor, Michigan Abstract 2Department of Biology, University of 1. The relationship between food-web structure (i.e., trophic connections, including Toronto, Mississauga, Ontario, Canada diet, trophic position, and habitat use, and the strength of these connections) and 3Department of Integrative ecosystem functions (i.e., biological, geochemical, and physical processes in an Biology, University of Guelph, Guelph, Ontario, Canada ecosystem, including decomposition, production, nutrient cycling, and nutrient 4Great Lakes Institute for Environmental and energy flows among community members) determines how an ecosystem re- Research, University of Windsor, Windsor, Ontario, Canada sponds to perturbations, and thus is key to understanding the adaptive capacity of 5Glenora Fisheries Station, Ontario Ministry a system (i.e., ability to respond to perturbation without loss of essential func- of Natural Resources and Forestry, Picton, tions). Given nearly ubiquitous changing environmental conditions and anthropo- Ontario, Canada genic impacts on global lake ecosystems, understanding the adaptive capacity of 6US Geological Survey Great Lakes Science Center, Ann Arbor, Michigan food webs supporting important resources, such as commercial, recreational, and 7Department of Fisheries and subsistence fisheries, is vital to ecological and economic stability. Oceans, Bedford Institute of Oceanography, Ocean Sciences Division, 2. Herein, we describe a conceptual framework that can be used to explore food- Dartmouth, Nova Scotia, Canada web structure and associated ecosystem functions in large lakes. -

Distribution Changes of Small Fishes in Streams of Missouri from The
Distribution Changes of Small Fishes in Streams of Missouri from the 1940s to the 1990s by MATTHEW R. WINSTON Missouri Department of Conservation, Columbia, MO 65201 February 2003 CONTENTS Page Abstract……………………………………………………………………………….. 8 Introduction…………………………………………………………………………… 10 Methods……………………………………………………………………………….. 17 The Data Used………………………………………………………………… 17 General Patterns in Species Change…………………………………………... 23 Conservation Status of Species……………………………………………….. 26 Results………………………………………………………………………………… 34 General Patterns in Species Change………………………………………….. 30 Conservation Status of Species……………………………………………….. 46 Discussion…………………………………………………………………………….. 63 General Patterns in Species Change………………………………………….. 53 Conservation Status of Species………………………………………………. 63 Acknowledgments……………………………………………………………………. 66 Literature Cited……………………………………………………………………….. 66 Appendix……………………………………………………………………………… 72 FIGURES 1. Distribution of samples by principal investigator…………………………. 20 2. Areas of greatest average decline…………………………………………. 33 3. Areas of greatest average expansion………………………………………. 34 4. The relationship between number of basins and ……………………….. 39 5. The distribution of for each reproductive group………………………... 40 2 6. The distribution of for each family……………………………………… 41 7. The distribution of for each trophic group……………...………………. 42 8. The distribution of for each faunal region………………………………. 43 9. The distribution of for each stream type………………………………… 44 10. The distribution of for each range edge…………………………………. 45 11. Modified -
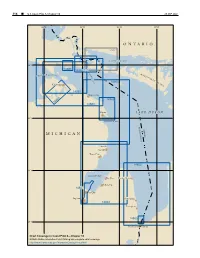
M I C H I G a N O N T a R
314 ¢ U.S. Coast Pilot 6, Chapter 10 26 SEP 2021 85°W 84°W 83°W 82°W ONTARIO 2251 NORTH CHANNEL 46°N D E 14885 T 14882 O U R M S O F M A C K I N A A I T A C P S T R N I A T O S U S L A I N G I S E 14864 L A N Cheboygan D 14881 Rogers City 14869 14865 14880 Alpena L AKE HURON 45°N THUNDER BAY UNITED ST CANADA MICHIGAN A TES Oscoda Au Sable Tawas City 14862 44°N SAGINAW BAY Bay Port Harbor Beach Sebewaing 14867 Bay City Saginaw Port Sanilac 14863 Lexington 14865 43°N Port Huron Sarnia Chart Coverage in Coast Pilot 6—Chapter 10 NOAA’s Online Interactive Chart Catalog has complete chart coverage http://www.charts.noaa.gov/InteractiveCatalog/nrnc.shtml 26 SEP 2021 U.S. Coast Pilot 6, Chapter 10 ¢ 315 Lake Huron (1) Lawrence, Great Lakes, Lake Winnipeg and Eastern Chart Datum, Lake Huron Arctic for complete information.) (2) Depths and vertical clearances under overhead (12) cables and bridges given in this chapter are referred to Fluctuations of water level Low Water Datum, which for Lake Huron is on elevation (13) The normal elevation of the lake surface varies 577.5 feet (176.0 meters) above mean water level at irregularly from year to year. During the course of each Rimouski, QC, on International Great Lakes Datum 1985 year, the surface is subject to a consistent seasonal rise (IGLD 1985).