Reintroduction of Fishes in Canada: a Review of Research Progress for SARA-Listed Species
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

2016 Lakewide Assessment Plan Survey Report
2016 Lakewide Assessment Plan Survey Report Report Number: 2017-04 Suggested Citation: Breeggemann, J. J., T. J. Treska, S. D. Hanson, and R. M. Wehse. 2017. 2016 Lakewide Assessment Plan Survey Report. Green Bay Fish and Wildlife Conservation Office Report number: 2017-04. March 2017 Table of Contents Introduction .................................................................................................................................................. 4 Methods ........................................................................................................................................................ 6 Field Sampling ........................................................................................................................................... 6 Lab Processing ........................................................................................................................................... 6 Data Analyses ............................................................................................................................................ 7 Results ........................................................................................................................................................... 8 Discussion ................................................................................................................................................... 10 References ................................................................................................................................................. -

Endangered Species
FEATURE: ENDANGERED SPECIES Conservation Status of Imperiled North American Freshwater and Diadromous Fishes ABSTRACT: This is the third compilation of imperiled (i.e., endangered, threatened, vulnerable) plus extinct freshwater and diadromous fishes of North America prepared by the American Fisheries Society’s Endangered Species Committee. Since the last revision in 1989, imperilment of inland fishes has increased substantially. This list includes 700 extant taxa representing 133 genera and 36 families, a 92% increase over the 364 listed in 1989. The increase reflects the addition of distinct populations, previously non-imperiled fishes, and recently described or discovered taxa. Approximately 39% of described fish species of the continent are imperiled. There are 230 vulnerable, 190 threatened, and 280 endangered extant taxa, and 61 taxa presumed extinct or extirpated from nature. Of those that were imperiled in 1989, most (89%) are the same or worse in conservation status; only 6% have improved in status, and 5% were delisted for various reasons. Habitat degradation and nonindigenous species are the main threats to at-risk fishes, many of which are restricted to small ranges. Documenting the diversity and status of rare fishes is a critical step in identifying and implementing appropriate actions necessary for their protection and management. Howard L. Jelks, Frank McCormick, Stephen J. Walsh, Joseph S. Nelson, Noel M. Burkhead, Steven P. Platania, Salvador Contreras-Balderas, Brady A. Porter, Edmundo Díaz-Pardo, Claude B. Renaud, Dean A. Hendrickson, Juan Jacobo Schmitter-Soto, John Lyons, Eric B. Taylor, and Nicholas E. Mandrak, Melvin L. Warren, Jr. Jelks, Walsh, and Burkhead are research McCormick is a biologist with the biologists with the U.S. -

Traceability Study in Shark Products
Traceability study in shark products Dr Heiner Lehr (Photo: © Francisco Blaha, 2015) Report commissioned by the CITES Secretariat This publication was funded by the European Union, through the CITES capacity-building project on aquatic species Contents 1 Summary.................................................................................................................................. 7 1.1 Structure of the remaining document ............................................................................. 9 1.2 Acknowledgements ....................................................................................................... 10 2 The market chain ................................................................................................................... 11 2.1 Shark Products ............................................................................................................... 11 2.1.1 Shark fins ............................................................................................................... 12 2.1.2 Shark meat ............................................................................................................. 12 2.1.3 Shark liver oil ......................................................................................................... 13 2.1.4 Shark cartilage ....................................................................................................... 13 2.1.5 Shark skin .............................................................................................................. -

DISTRIBUTION, ECOLOGY, and REPRODUCTIVE BIOLOGY of the ORANGEFIN MADTOM (NOTURUS GILBERTI) by Timothy Dale Simonson
DISTRIBUTION, ECOLOGY, AND REPRODUCTIVE BIOLOGY OF THE ORANGEFIN MADTOM (NOTURUS GILBERTI) by Timothy Dale Simonson Thesis submitted to the Faculty of the Virginia Polytechnic Institute and State University in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE in Fisheries and Wildlife Sciences APPROVED: Richard J. Neves, Chair Dona:;[d J. Orth Johm J. Ney Louis A. Hel:frich April 1987 Blacksburg, Virginia DISTRIBUTION, ECOLOGY, AND REPRODUCTIVE BIOLOGY OF THE ORANGEFIN MADTOM (NOTURUS GILBERTI) by Timothy Dale Simonson Richard J. Neves, Chair Fisheries and Wildlife Sciences (ABSTRACT) Distribution of the orangefin madtom (Noturus gilberti) was determined from 347 sites sampled in Virginia and North Carolina. This species inhabited 264 stream kilometers, over twice the reported range, in the following systems: Craig Creek, Roanoke River, Dan River, Big Chestnut Creek, South Mayo River, Pigg River, and Smith River. The orangefin madtom was somewhat common; 33% (Dan River) to 70% (Craig Creek) of the sites sampled were occupied. Negative interspecific associates of orangefin madtoms included chubs, mountain redbelly dace, rosyside dace, crescent shiners, and crayfish; only Roanoke darters were considered positive associates. Sand and silt levels were significantly lower at sites with !L. gilberti, while per- centage of small cobble, local gradient, and depth were sig- nificantly higher. Discriminant function analysis identified large gravel, local gradient, silt, and occurrence of rosyside dace and crayfish, as significant predictors of the occurrence of the orangefin madtom. Seasonal samples from Craig Creek consisted of three age groups. The smallest individual captured was 33 mm total length (TL) and the largest was 111 mm TL. -

(Coregonus Zenithicus) in Lake Superior
Ann. Zool. Fennici 41: 147–154 ISSN 0003-455X Helsinki 26 February 2004 © Finnish Zoological and Botanical Publishing Board 2004 Status of the shortjaw cisco (Coregonus zenithicus) in Lake Superior Michael H. Hoff1 & Thomas N. Todd2* 1) U.S. Geological Survey, Great Lakes Science Center, 2800 Lake Shore Drive East, Ashland, Wisconsin 54806, USA; present address: U.S. Fish and Wildlife Service, Fisheries Division, Federal Building, 1 Federal Drive, Ft. Snelling, Minnesota 55111, USA. 2) U.S. Geological Survey, Great Lakes Science Center, 1451 Green Road, Ann Arbor, Michigan 48105, USA (*corresponding author) Received 26 Aug. 2002, revised version received 7 Mar. 2003, accepted 9 Sep. 2003 Hoff, M. H. & Todd, T. N. 2004: Status of the shortjaw cisco (Coregonus zenithicus) in Lake Supe- rior. — Ann. Zool. Fennici 41: 147–154. The shortjaw cisco (Coregonus zenithicus) was historically found in Lakes Huron, Michigan, and Superior, but has been extirpated in Lakes Huron and Michigan appar- ently as the result of commercial overharvest. During 1999–2001, we conducted an assessment of shortjaw cisco abundance in fi ve areas, spanning the U.S. waters of Lake Superior, and compared our results with the abundance measured at those areas in 1921–1922. The shortjaw cisco was found at four of the fi ve areas sampled, but abundances were so low that they were not signifi cantly different from zero. In the four areas where shortjaw ciscoes were found, abundance declined signifi cantly by 99% from the 1920s to the present. To increase populations of this once economically and ecologically important species in Lake Superior, an interagency rehabilitation effort is needed. -

Lake Superior Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake Superior Food Web MENT OF C Sea Lamprey Walleye Burbot Lake Trout Chinook Salmon Brook Trout Rainbow Trout Lake Whitefish Bloater Yellow Perch Lake herring Rainbow Smelt Deepwater Sculpin Kiyi Ruffe Lake Sturgeon Mayfly nymphs Opossum Shrimp Raptorial waterflea Mollusks Amphipods Invasive waterflea Chironomids Zebra/Quagga mussels Native waterflea Calanoids Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake Superior, 2009. 734-741-2235 - www.glerl.noaa.gov Lake Superior Food Web Sea Lamprey Macroinvertebrates Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Chironomids/Oligochaetes. Larval insects and worms that live on the lake fastens onto its prey and rasps out a hole with its rough tongue. bottom. Feed on detritus. Species present are a good indicator of water quality. Piscivores (Fish Eaters) Amphipods (Diporeia). The most common species of amphipod found in fish diets that began declining in the late 1990’s. Chinook salmon (Oncorhynchus tshawytscha). Pacific salmon species stocked as a trophy fish and to control alewife. Opossum shrimp (Mysis relicta). An omnivore that feeds on algae and small cladocerans. -

Spawning Distribution of Bering Ciscoes in the Yukon River
Transactions of the American Fisheries Society 144:292–299, 2015 American Fisheries Society 2015 ISSN: 0002-8487 print / 1548-8659 online DOI: 10.1080/00028487.2014.988881 ARTICLE Spawning Distribution of Bering Ciscoes in the Yukon River Randy J. Brown* and David W. Daum1 U.S. Fish and Wildlife Service, 101 12th Avenue, Room 110, Fairbanks, Alaska 99701, USA Abstract Bering Ciscoes Coregonus laurettae are anadromous salmonids with known spawning populations only in the Yukon, Kuskokwim, and Susitna rivers in Alaska. A commercial fishery for the species was recently initiated at the mouth of the Yukon River, inspiring a series of research projects to enhance our understanding of the exploited population. This study was designed to delineate the geographic spawning distribution of Bering Ciscoes in the Yukon River. One hundred radio transmitters per year in 2012 and 2013 were deployed in prespawning Bering Ciscoes at a site located 1,176 km upstream from the sea. A total of 160 fish survived fish wheel capture and tagging, avoided harvest and predation after tagging, and continued migrating upstream to their spawning destinations. Approximately 79% migrated to spawn in the upper Yukon Flats, upstream from the mouth of the Porcupine River, and 21% migrated to spawn in the lower Yukon Flats. Locating the Bering Cisco spawning area, which is almost entirely encompassed by the Yukon Flats National Wildlife Refuge, enhances our ability to protect it from anthropogenic disturbance and enables future biological research on the spawning population. Conservation of migratory fish in large rivers requires an removing gravel from fish spawning habitats has been shown understanding of habitat use across a species’ range and the to reduce spawning success (Fudge and Bodaly 1984; Meng ability to manage anthropogenic impacts to essential habitats and Muller€ 1988), which could jeopardize the viability of such as migration routes and spawning areas (Gross 1987; affected populations. -

Ours to Save: the Distribution, Status & Conservation Needs of Canada's Endemic Species
Ours to Save The distribution, status & conservation needs of Canada’s endemic species June 4, 2020 Version 1.0 Ours to Save: The distribution, status & conservation needs of Canada’s endemic species Additional information and updates to the report can be found at the project website: natureconservancy.ca/ourstosave Suggested citation: Enns, Amie, Dan Kraus and Andrea Hebb. 2020. Ours to save: the distribution, status and conservation needs of Canada’s endemic species. NatureServe Canada and Nature Conservancy of Canada. Report prepared by Amie Enns (NatureServe Canada) and Dan Kraus (Nature Conservancy of Canada). Mapping and analysis by Andrea Hebb (Nature Conservancy of Canada). Cover photo credits (l-r): Wood Bison, canadianosprey, iNaturalist; Yukon Draba, Sean Blaney, iNaturalist; Salt Marsh Copper, Colin Jones, iNaturalist About NatureServe Canada A registered Canadian charity, NatureServe Canada and its network of Canadian Conservation Data Centres (CDCs) work together and with other government and non-government organizations to develop, manage, and distribute authoritative knowledge regarding Canada’s plants, animals, and ecosystems. NatureServe Canada and the Canadian CDCs are members of the international NatureServe Network, spanning over 80 CDCs in the Americas. NatureServe Canada is the Canadian affiliate of NatureServe, based in Arlington, Virginia, which provides scientific and technical support to the international network. About the Nature Conservancy of Canada The Nature Conservancy of Canada (NCC) works to protect our country’s most precious natural places. Proudly Canadian, we empower people to safeguard the lands and waters that sustain life. Since 1962, NCC and its partners have helped to protect 14 million hectares (35 million acres), coast to coast to coast. -

(Acipenser Brevirostrum) in Canada Shortnose Sturgeon
Proposed Species at Risk Act Management Plan Series Management Plan for the Shortnose Sturgeon (Acipenser brevirostrum) in Canada Shortnose Sturgeon 2015 Management Plan for the Shortnose Sturgeon [Proposed] 2015 Recommended citation: Draft Fisheries and Oceans Canada. 2015. Management Plan for the Shortnose Sturgeon (Acipenser brevirostrum) in Canada [Proposed]. Species at Risk Act Management Plan Series. Fisheries and Oceans Canada, Ottawa. v + 55 pp. Additional copies: For copies of the Management Plan, or for additional information on species at risk, including COSEWIC Status Reports, residence descriptions, action plans, and other related recovery documents, please visit the SAR Public Registry. Cover illustration: DFO (2007) Également disponible en français sous le titre « Plan de gestion pour l’esturgeon à museau court (Acipenser brevirostrum) au Canada» © Her Majesty the Queen in Right of Canada, represented by the Minister of the Environment, 2015. All rights reserved. ISBN ISBN to be included Catalogue no. Catalogue no. to be included Content (excluding the illustrations) may be used without permission, with appropriate credit to the source. Management Plan for the Shortnose Sturgeon [Proposed] 2015 Preface Under the Species at Risk Act (S.C. 2002, c.29) (SARA), the federal competent ministers are responsible for the preparation of Management Plans for listed species of Special Concern and are required to report on progress within five years. The federal, provincial, and territorial government signatories under the Accord for the Protection of Species at Risk (1996) agreed to establish complementary legislation and programs that provide for effective protection of species at risk throughout Canada. The Minister of Fisheries and Oceans is the competent minister under SARA for the Shortnose Sturgeon and has prepared this Management Plan as per section 65 of SARA. -

September 24, 2018
September 24, 2018 Sent via Federal eRulemaking Portal to: http://www.regulations.gov Docket Nos. FWS-HQ-ES-2018-0006 FWS-HQ-ES-2018-0007 FWS-HQ-ES-2018-0009 Bridget Fahey Chief, Division of Conservation and Classification U.S. Fish and Wildlife Service 5275 Leesburg Pike, MS: ES Falls Church, VA 22041-3808 [email protected] Craig Aubrey Chief, Division of Environmental Review Ecological Services Program U.S. Fish and Wildlife Service 5275 Leesburg Pike, MS: ES Falls Church, VA 22041 [email protected] Samuel D. Rauch, III National Marine Fisheries Service Office of Protected Resources 1315 East-West Highway Silver Spring, MD 20910 [email protected] Re: Proposed Revisions of Endangered Species Act Regulations Dear Mr. Aubrey, Ms. Fahey, and Mr. Rauch: The Southern Environmental Law Center (“SELC”) submits the following comments in opposition to the U.S. Fish and Wildlife Service’s and National Marine Fisheries Service’s proposed revisions to the Endangered Species Act’s implementing regulations.1 We submit these comments on behalf of 57 organizations working to protect the natural resources of the 1 Revision of the Regulations for Prohibitions to Threatened Wildlife and Plants, 83 Fed. Reg. 35,174 (proposed July 25, 2018) (to be codified at 50 C.F.R. pt. 17); Revision of Regulations for Interagency Cooperation, 83 Fed. Reg. 35,178 (proposed July 25, 2018) (to be codified at 50 C.F.R. pt. 402); Revision of the Regulations for Listing Species and Designating Critical Habitat, 83 Fed. Reg. 35,193 (proposed July 25, 2018) (to be codified at 50 C.F.R. -
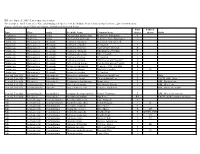
Threatened and Endangered Species List
Effective April 15, 2009 - List is subject to revision For a complete list of Tennessee's Rare and Endangered Species, visit the Natural Areas website at http://tennessee.gov/environment/na/ Aquatic and Semi-aquatic Plants and Aquatic Animals with Protected Status State Federal Type Class Order Scientific Name Common Name Status Status Habit Amphibian Amphibia Anura Gyrinophilus gulolineatus Berry Cave Salamander T Amphibian Amphibia Anura Gyrinophilus palleucus Tennessee Cave Salamander T Crustacean Malacostraca Decapoda Cambarus bouchardi Big South Fork Crayfish E Crustacean Malacostraca Decapoda Cambarus cymatilis A Crayfish E Crustacean Malacostraca Decapoda Cambarus deweesae Valley Flame Crayfish E Crustacean Malacostraca Decapoda Cambarus extraneus Chickamauga Crayfish T Crustacean Malacostraca Decapoda Cambarus obeyensis Obey Crayfish T Crustacean Malacostraca Decapoda Cambarus pristinus A Crayfish E Crustacean Malacostraca Decapoda Cambarus williami "Brawley's Fork Crayfish" E Crustacean Malacostraca Decapoda Fallicambarus hortoni Hatchie Burrowing Crayfish E Crustacean Malocostraca Decapoda Orconectes incomptus Tennessee Cave Crayfish E Crustacean Malocostraca Decapoda Orconectes shoupi Nashville Crayfish E LE Crustacean Malocostraca Decapoda Orconectes wrighti A Crayfish E Fern and Fern Ally Filicopsida Polypodiales Dryopteris carthusiana Spinulose Shield Fern T Bogs Fern and Fern Ally Filicopsida Polypodiales Dryopteris cristata Crested Shield-Fern T FACW, OBL, Bogs Fern and Fern Ally Filicopsida Polypodiales Trichomanes boschianum -

Habitat Evaluation, Restauration and Education for the Salmonid Populations in the Shediac Bay Watershed
Habitat Evaluation, Restauration and Education for the Salmonid populations in the Shediac Bay Watershed Prepared for: New Brunswick Wildlife Trust Fund Atlantic Salmon Conservation Foundation DFO Recreational Fisheries Partnership Program Prepared by: Shediac Bay Watershed Association Jolyne Hébert November 30th, 2015 Table of Contents Abstract.......................................................................................................................................................................... v 1. Introduction ........................................................................................................................................................... 1 2. Project results ........................................................................................................................................................ 2 2.1 Electrofishing Surveys ......................................................................................................................................... 2 2.2 Electrofishing Sites Descriptions and Results ..................................................................................................... 3 2.2.1 Scotch Settlement (EShdB-01) ..................................................................................................................... 4 2.2.2 Dionne Brook (EScdB-01)............................................................................................................................ 6 2.2.3 Pellerin Road (EScdF-01) ............................................................................................................................