Status of Burbot Populations in the Laurentian Great Lakes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Ecology and Management of the European Grayling Thymallus Thymallus (Linnaeus)
The ecology and management of the European grayling Thymallus thymallus (Linnaeus). Interim report Item Type monograph Authors Ingram, A.; Ibbotson, A.; Gallagher, M. Publisher Institute of Freshwater Ecology Download date 03/10/2021 22:03:11 Link to Item http://hdl.handle.net/1834/24874 The Ecology and Management of the European Grayling Thymallus thymallus (Linnaeus) Interim Report Ingram A Ibbotson A Gallagher M The Ecology and Management of the European Grayling Thymallus thymallus (Linnaeus) Interim Report Ingram A Ibbotson A Gallagher M INTELLECTUAL PROPERTY RIGHTS CONFIDENTIALITY STATEMENT 'In accordance with our normal practice, this report is for the use only of the party to whom it is addressed, and no responsibility is accepted to any third party for the whole or any part of its contents. Neither the whole nor any part of this report or any reference thereto may be included in any published document, circular or statement, nor published or referred to in any way without our written approval of the form and context in which it may appear.' 11 CHAPTER 1 1 Overall aim A collaborative research and development project between the Institute of Freshwater Ecology and the Environment Agency in the UK, to review the ecology, status and management of grayling in order to provide recommendations for future management of grayling fisheries in England and Wales. 2 Objectives • To review grayling ecology, status and management practice in concentrating on England and Wales but including published literature from Europe and North America, where appropriate. • To recommend best management practices on the basis of objective 1 and produce a guidance leaflet for internal and external circulation which promotes the key issues. -

Burbot Management Plan
BURBOT MANAGEMENT PLAN DEPARTMENT OF INLAND FISHERIES AND WILDLIFE DIVISION OF FISHERIES AND HATCHERIES PREPARED BY SCOTT A. ROY ASSISTANT REGIONAL FISHERIES BIOLOGIST REGION E MARCH 2001 BURBOT LIFE HISTORY The burbot, Lota lota (Linnaeus), is a unique member of the cod family. It is the only species in the family, which spends its entire life in fresh water. However, the burbot is similar to its marine relatives in that its distribution is circumpolar. It can be found in cool, fresh waters throughout northern Europe, Asia and North America. In North America its range extends as far south as the northern tier of States across the United States. In Maine, the burbot is commonly known as the cusk, although in other areas it is also called the ling, eelpout, loche and lawyer. Unlike the salmonids and Maine’s other coldwater species, the burbot is not noted for its grace and beauty. The body is elongate, almost eel-shaped, with long, soft-rayed dorsal and anal fins that meet a rounded tail. Although it is smooth and slimy to the touch, the skin is embedded with very small, cycloid scales. The head of the burbot is broad and somewhat flattened. It has a large mouth containing several rows of small teeth on the jaws. A single, whisker-like barbell protrudes from the tip of the chin. There are no obvious external differences between males and females. In general, adults are olive brown to dark brown on the back and sides. This background is overlaid with distinctive patterns of dark brown or black markings and spots. -

Edna Assay Development
Environmental DNA assays available for species detection via qPCR analysis at the U.S.D.A Forest Service National Genomics Center for Wildlife and Fish Conservation (NGC). Asterisks indicate the assay was designed at the NGC. This list was last updated in June 2021 and is subject to change. Please contact [email protected] with questions. Family Species Common name Ready for use? Mustelidae Martes americana, Martes caurina American and Pacific marten* Y Castoridae Castor canadensis American beaver Y Ranidae Lithobates catesbeianus American bullfrog Y Cinclidae Cinclus mexicanus American dipper* N Anguillidae Anguilla rostrata American eel Y Soricidae Sorex palustris American water shrew* N Salmonidae Oncorhynchus clarkii ssp Any cutthroat trout* N Petromyzontidae Lampetra spp. Any Lampetra* Y Salmonidae Salmonidae Any salmonid* Y Cottidae Cottidae Any sculpin* Y Salmonidae Thymallus arcticus Arctic grayling* Y Cyrenidae Corbicula fluminea Asian clam* N Salmonidae Salmo salar Atlantic Salmon Y Lymnaeidae Radix auricularia Big-eared radix* N Cyprinidae Mylopharyngodon piceus Black carp N Ictaluridae Ameiurus melas Black Bullhead* N Catostomidae Cycleptus elongatus Blue Sucker* N Cichlidae Oreochromis aureus Blue tilapia* N Catostomidae Catostomus discobolus Bluehead sucker* N Catostomidae Catostomus virescens Bluehead sucker* Y Felidae Lynx rufus Bobcat* Y Hylidae Pseudocris maculata Boreal chorus frog N Hydrocharitaceae Egeria densa Brazilian elodea N Salmonidae Salvelinus fontinalis Brook trout* Y Colubridae Boiga irregularis Brown tree snake* -

Sport-Fish-Identification.Pdf
Walleye Walleye have two distinct fins on their back, the first with large spines. Lake Sturgeon They have a yellow-olive back, brassy, silvery sides with yellow spots, a white underside, and white on the lower lobe of the tail. Dusky vertical Lake Sturgeon are a Threatened Species due to population size and bars are often found on the body as well. concerns with viability. Lake Sturgeon have a large brown or grey body covered with tough, leather- like tissue and five rows of bony plates. They have a shark-like, upturned tail and a pointed snout with four barbels. Sauger Lake Whitefish are olive-green to blue on the back, with silvery sides.They Sauger are a Threatened Species due to hybridization, habitat Lakehave a small Whitefish mouth below a rounded snout, and a deeply forked tail. degradation and overharvest. Sauger are golden olive on the back with silver-yellow sides and a white underside. They also have a large spiny dorsal fin, distinct rows of spots on the dorsal fins and three or four dusky vertical bars on the body. Mountain Whitefish have large scales, no spots and small mouths with no Burbot Mountainteeth. Their general Whitefish body colour is a bronze-white or greenish white. Burbot have a slim, brownish black body with smooth skin, a flattened head, and a fin that stretches along the back half of the body. Distinctive barbels hang from the lower jaw and nostrils. Goldeye Northern Pike Goldeye have prominent eyes with bright yellow pupils, a blunt head, and Northern Pike are a long, slender fish with duck-like jaws and a long, flat a deep, compressed body. -
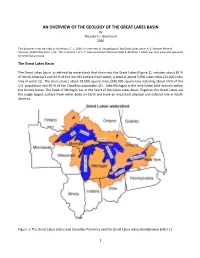
AN OVERVIEW of the GEOLOGY of the GREAT LAKES BASIN by Theodore J
AN OVERVIEW OF THE GEOLOGY OF THE GREAT LAKES BASIN by Theodore J. Bornhorst 2016 This document may be cited as: Bornhorst, T. J., 2016, An overview of the geology of the Great Lakes basin: A. E. Seaman Mineral Museum, Web Publication 1, 8p. This is version 1 of A. E. Seaman Mineral Museum Web Publication 1 which was only internally reviewed for technical accuracy. The Great Lakes Basin The Great Lakes basin, as defined by watersheds that drain into the Great Lakes (Figure 1), includes about 85 % of North America’s and 20 % of the world’s surface fresh water, a total of about 5,500 cubic miles (23,000 cubic km) of water (1). The basin covers about 94,000 square miles (240,000 square km) including about 10 % of the U.S. population and 30 % of the Canadian population (1). Lake Michigan is the only Great Lake entirely within the United States. The State of Michigan lies at the heart of the Great Lakes basin. Together the Great Lakes are the single largest surface fresh water body on Earth and have an important physical and cultural role in North America. Figure 1: The Great Lakes states and Canadian Provinces and the Great Lakes watershed (brown) (after 1). 1 Precambrian Bedrock Geology The bedrock geology of the Great Lakes basin can be subdivided into rocks of Precambrian and Phanerozoic (Figure 2). The Precambrian of the Great Lakes basin is the result of three major episodes with each followed by a long period of erosion (2, 3). Figure 2: Generalized Precambrian bedrock geologic map of the Great Lakes basin. -

Westslope Cutthroat Trout
This file was created by scanning the printed publication. Errors identified by the software have been corrected; Chapter 1 however, some errors may remain. Westslope Cutthroat Trout John D. IVIclntyre and Bruce E. Rieman, USDA Forest Service, Intermountain Research Station, 316 E. iViyrtle Street, Boise, Idaho 83702 Introduction Westslope cutthroat trout begin to mature at age 3 but usually spawn first at age 4 or 5 (table 2). Sexu The westslope cutthroat trout inhabits streams on ally maturing adfluvial fish move into the vicinity of both sides of the Continental Divide. On the east side tributaries in fall and winter where they remain un of the divide, they are distributed mostly in Mon til they begin to migrate upstream in the spring tana but also occur in some headwaters in Wyoming (Liknes 1984). They spawn from March to July at and southern Alberta (Behnke 1992). They are in the water temperatures near 10°C (Roscoe 1974; Liknes Missouri Basin downstream to about 60 km below 1984; Shepard et al. 1984). A population of adult fish Great Falls and in the headwaters of the Judith, Milk, in the St. Joe River, Idaho, included 1.6 females for and Marias rivers. On the west side of the Continen each male (Thurow and Bjornn 1978). Average length tal Divide the subspecies occurs in the upper was 334 mm for females and 366 mm for males. A Kootenai River; the Clark Fork drainage in Montana similar population in Big Creek, Montana, included and Idaho downstream to the falls on the Fend Oreille 4.1 females for each male (Huston et al. -

Nipigon Bay Area of Concern Status of Beneficial Use Impairments September 2010
Nipigon Bay Area of Concern Status of Beneficial Use Impairments September 2010 Nipigon Bay is in the most northerly area of Lake Superior. The Area of Concern takes in a large portion of Nipigon Bay and the Nipigon River, the largest tributary to Lake Superior, and the communities of Red Rock and Nipigon. There are Ontario Power Generation dams on the Nipigon River for the generation of hydroelectricity. The area supports a variety of wetlands and bird populations, including one of four known pelican colonies in Ontario. The watershed forests on both sides of the Nipigon River have been allocated for forest harvesting. Environmental concerns in the Nipigon Bay Area of Concern are related to water level and flow fluctuations in Lake Nipigon and the Nipigon River from the generation of hydroelectricity. These fluctuations affect stream bank stability, sediment load and fish and wildlife habitat. Other concerns include the accumulation of wood fibre, bark and other organic material from past log drives, and effluent discharges from a linerboard mill (which closed in 2006) and the municipal sewage treatment plants in Nipigon and Red Rock. PARTNERSHIPS IN ENVIRONMENTAL PROTECTION Nipigon Bay was designated an Area of Concern in 1987 under the Canada–United States Great Lakes Water Quality Agreement. Areas of Concern are sites on the Great Lakes system where environmental quality is significantly degraded and beneficial uses are impaired. Currently, there are 9 such designated areas on the Canadian side of the Great Lakes, 25 in the United States, and 5 that are shared by both countries. In each Area of Concern, government, community and industry partners are undertaking a coordinated effort to restore environmental quality and beneficial uses through a remedial action plan. -

1 WYOMING GAME and FISH DEPARTMENT March 1, 2013
GOVERNOR MATTHEW H. MEAD DIRECTOR WYOMING GAME AND FISH DEPARTMENT SCOTT TALBOTT COMMISSIONERS AARON CLARK – President 5400 Bishop Blvd. Cheyenne, WY 82006 MIKE HEALY – Vice President 1 MARK ANSELMI Phone: (307) 777-4600 Fax: (307) 777-4699 KEITH CULVER RICHARD KLOUDA wgfd.wyo.gov T. CARRIE LITTLE CHARLES PRICE March 1, 2013 MEMORANDUM TO: Honorable Matthew H. Mead FROM: Mark Fowden, Chief, Fish Division COPY TO: Director’s Office, Mike Choma, file SUBJECT: Justification for Emergency Rule, Chapter 46, Fishing Regulations The Sixty-Second Legislature of the State of Wyoming 2013 General Session made a change to W.S. §23-1-101. This change was included in HB 0131/HEA 0052. Specifically, the provision enabled the Wyoming Game and Fish Commission to designate game fish as nongame fish in specific waters. This statutory change was intended to become effective immediately. Due to the Department’s cycle for updating fishing regulations only once every two years, an emergency rule is necessary in order to have this change effective prior to January 1, 2014. The Wyoming Game and Fish Department proposes to amend Wyoming Game and Fish Commission Chapter 46, Fishing Regulations, to edit the definition of “Game Fish” to allow a game fish to be designated as a nongame fish in specific waters. The Department also proposes to designate a game fish, burbot (ling), as a nongame fish in the Green River, Little Snake River, Bear River and Great Divide Basin drainages. This regulation change will allow anglers harvesting burbot in these drainages to dispose of edible portions of this illegally introduced species. -

Lake Superior Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake Superior Food Web MENT OF C Sea Lamprey Walleye Burbot Lake Trout Chinook Salmon Brook Trout Rainbow Trout Lake Whitefish Bloater Yellow Perch Lake herring Rainbow Smelt Deepwater Sculpin Kiyi Ruffe Lake Sturgeon Mayfly nymphs Opossum Shrimp Raptorial waterflea Mollusks Amphipods Invasive waterflea Chironomids Zebra/Quagga mussels Native waterflea Calanoids Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake Superior, 2009. 734-741-2235 - www.glerl.noaa.gov Lake Superior Food Web Sea Lamprey Macroinvertebrates Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Chironomids/Oligochaetes. Larval insects and worms that live on the lake fastens onto its prey and rasps out a hole with its rough tongue. bottom. Feed on detritus. Species present are a good indicator of water quality. Piscivores (Fish Eaters) Amphipods (Diporeia). The most common species of amphipod found in fish diets that began declining in the late 1990’s. Chinook salmon (Oncorhynchus tshawytscha). Pacific salmon species stocked as a trophy fish and to control alewife. Opossum shrimp (Mysis relicta). An omnivore that feeds on algae and small cladocerans. -

2018 Roadmap to Fishing Lake Superior: Have a Super Time!
Roadmap to Fishing Lake Superior: Have a Super Time! Want to fish Lake Superior, but aren’t sure when or where to go? This roadmap is a starting point to provide you with information on many fishing sites and times of the year when great opportunities exist. Please note this is not an exhaustive list. Eagle HarborCopper Harbor Traverse Bay Ontonagon Keweenaw Bay Black River Harbor Grand Marais Marquette Au Train-Shelter Bay St. Marys River Munising Black River Au Train- Ontonagon Eagle Harbor Copper Harbor Traverse Bay Keweenaw Bay Marquette Munising Grand Marais St. Marys River Port Harbor Shelter Bay brown trout, Chinook, cisco, coho, brown trout, Chinook, cisco, brown trout, Chinook, cisco, coho, brown trout, lake trout, lake whitefish, round coho, lake trout, lake whitefish, lake trout, lake whitefish, round coho, lake whitefish, n burbot, coho, cisco, walleye, yellow perch Feb - March whitefish, siscowet, smelt, splake, round whitefish, siscowet, smelt, whitefish, splake, steelhead, pike, yellow perch smelt, steelhead steelhead, yellow perch splake, steelhead, yellow perch yellow perch brown trout, Chinook, coho, lake brown trout, Chinook, coho, brown trout, Chinook, cisco, Chinook, cisco, coho, lake brown trout, Chinook, brown trout, Chinook, cisco, coho, Chinook, trout, lake whitefish, Chinook, coho, lake trout, coho, lake trout, round trout, lake whitefish, n pike, coho, lake trout, lake siscowet lake trout, lake whitefish, siscowet, coho, lake round whitefish, April - May lake trout, siscowet, whitefish, siscowet, smelt, sm bass, -

Differences in Brain Transcriptomes of Closely Related Baikal Coregonid Species
Differences in brain transcriptomes of closely related baikal coregonid species Bychenko, O. S., Sukhanova, L. V., Azhikina, T. L., Skvortsov, T. A., Belomestnykh, T. V., & Sverdlov, E. D. (2014). Differences in brain transcriptomes of closely related baikal coregonid species. BioMed Research International, 2014, [857329]. DOI: 10.1155/2014/857329 Published in: BioMed Research International Document Version: Publisher's PDF, also known as Version of record Queen's University Belfast - Research Portal: Link to publication record in Queen's University Belfast Research Portal Publisher rights Copyright 2017 the authors. This is an open access article published under a Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided the author and source are cited. General rights Copyright for the publications made accessible via the Queen's University Belfast Research Portal is retained by the author(s) and / or other copyright owners and it is a condition of accessing these publications that users recognise and abide by the legal requirements associated with these rights. Take down policy The Research Portal is Queen's institutional repository that provides access to Queen's research output. Every effort has been made to ensure that content in the Research Portal does not infringe any person's rights, or applicable UK laws. If you discover content in the Research Portal that you believe breaches copyright or violates any law, please contact [email protected]. Download date:06. Nov. 2017 Hindawi Publishing Corporation BioMed Research International Volume 2014, Article ID 857329, 11 pages http://dx.doi.org/10.1155/2014/857329 Research Article Differences in Brain Transcriptomes of Closely Related Baikal Coregonid Species Oksana S. -

Line 5 Straits of Mackinac Summary When Michigan Was Granted
Line 5 Straits of Mackinac Summary When Michigan was granted statehood on January 26, 1837, Michigan also acquired ownership of the Great Lakes' bottomlands under the equal footing doctrine.1 However before Michigan could become a state, the United States first had to acquire title from us (Ottawa and Chippewa bands) because Anglo-American law acknowledged that we owned legal title as the aboriginal occupants of the territory we occupied. But when we agreed to cede legal title to the United States in the March 28, 1836 Treaty of Washington ("1836 Treaty", 7 Stat. 491), we reserved fishing, hunting and gathering rights. Therefore, Michigan's ownership of both the lands and Great Lakes waters within the cession area of the 1836 Treaty was burdened with preexisting trust obligations with respect to our treaty-reserved resources. First, the public trust doctrine imposes a duty (trust responsibility) upon Michigan to protect the public trust in the resources dependent upon the quality of the Great Lakes water.2 In addition, Art. IV, § 52 of Michigan's Constitution says "conservation…of the natural resources of the state are hereby declared to be of paramount public concern…" and then mandates the legislature to "provide for the protection of the air, water and other natural resources from 3 pollution, impairment and destruction." 1 The State of Michigan acquired title to these bottomlands in its sovereign capacity upon admission to the Union and holds them in trust for the benefit of the people of Michigan. Illinois Central Railroad Co. v. Illinois, 146 U.S. 387, 434-35 (1892); Nedtweg v.