Modified Release Dosage Forms
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

An Overview On: Sublingual Route for Systemic Drug Delivery
International Journal of Research in Pharmaceutical and Biomedical Sciences ISSN: 2229-3701 __________________________________________Review Article An Overview on: Sublingual Route for Systemic Drug Delivery K. Patel Nibha1 and SS. Pancholi2* 1Department of Pharmaceutics, BITS Institute of Pharmacy, Gujarat Technological university, Varnama, Vadodara, Gujarat, India 2BITS Institute of Pharmacy, Gujarat Technological University, Varnama, Vadodara, Gujarat, India. __________________________________________________________________________________ ABSTRACT Oral mucosal drug delivery is an alternative and promising method of systemic drug delivery which offers several advantages. Sublingual literally meaning is ''under the tongue'', administrating substance via mouth in such a way that the substance is rapidly absorbed via blood vessels under tongue. Sublingual route offers advantages such as bypasses hepatic first pass metabolic process which gives better bioavailability, rapid onset of action, patient compliance , self-medicated. Dysphagia (difficulty in swallowing) is common among in all ages of people and more in pediatric, geriatric, psychiatric patients. In terms of permeability, sublingual area of oral cavity is more permeable than buccal area which is in turn is more permeable than palatal area. Different techniques are used to formulate the sublingual dosage forms. Sublingual drug administration is applied in field of cardiovascular drugs, steroids, enzymes and some barbiturates. This review highlights advantages, disadvantages, different sublingual formulation such as tablets and films, evaluation. Key Words: Sublingual delivery, techniques, improved bioavailability, evaluation. INTRODUCTION and direct access to systemic circulation, the oral Drugs have been applied to the mucosa for topical mucosal route is suitable for drugs, which are application for many years. However, recently susceptible to acid hydrolysis in the stomach or there has been interest in exploiting the oral cavity which are extensively metabolized in the liver. -

Liposome-Based Drug Delivery Systems in Cancer Immunotherapy
pharmaceutics Review Liposome-Based Drug Delivery Systems in Cancer Immunotherapy Zili Gu 1 , Candido G. Da Silva 1 , Koen van der Maaden 2,3, Ferry Ossendorp 2 and Luis J. Cruz 1,* 1 Department of Radiology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands 2 Tumor Immunology Group, Department of Immunology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, The Netherlands 3 TECOdevelopment GmbH, 53359 Rheinbach, Germany Received: 1 October 2020; Accepted: 2 November 2020; Published: 4 November 2020 Abstract: Cancer immunotherapy has shown remarkable progress in recent years. Nanocarriers, such as liposomes, have favorable advantages with the potential to further improve cancer immunotherapy and even stronger immune responses by improving cell type-specific delivery and enhancing drug efficacy. Liposomes can offer solutions to common problems faced by several cancer immunotherapies, including the following: (1) Vaccination: Liposomes can improve the delivery of antigens and other stimulatory molecules to antigen-presenting cells or T cells; (2) Tumor normalization: Liposomes can deliver drugs selectively to the tumor microenvironment to overcome the immune-suppressive state; (3) Rewiring of tumor signaling: Liposomes can be used for the delivery of specific drugs to specific cell types to correct or modulate pathways to facilitate better anti-tumor immune responses; (4) Combinational therapy: Liposomes are ideal vehicles for the simultaneous delivery of drugs to be combined with other therapies, including chemotherapy, radiotherapy, and phototherapy. In this review, different liposomal systems specifically developed for immunomodulation in cancer are summarized and discussed. Keywords: liposome; drug delivery; cancer immunotherapy; immunomodulation 1. The Potential of Immunotherapy for the Treatment of Cancer Cancer immunotherapy has been widely explored because of its durable and robust effects [1]. -

Intra-Luminal Focused Ultrasound for Augmentation of Gastrointestinal Drug Delivery
Editorial Page 1 of 2 Intra-luminal focused ultrasound for augmentation of gastrointestinal drug delivery Ezekiel Maloney1, Joo Ha Hwang2 1Department of Radiology, 2Division of Gastroenterology, Department of Medicine, University of Washington, Seattle, WA, USA Correspondence to: Joo Ha Hwang, MD, PhD. Division of Gastroenterology, Department of Medicine, University of Washington, Box 359773, 325 Ninth Avenue, Seattle, WA 98104, USA. Email: [email protected]. Provenance: This is a Guest Editorial commissioned by Section Editor Hui Kong, MD, PhD (Department of Respiratory Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China). Comment on: Schoellhammer CM, Schroeder A, Maa R, et al. Ultrasound-mediated gastrointestinal drug delivery. Sci Transl Med 2015;7:310ra168. Submitted Feb 01, 2017. Accepted for publication Feb 06, 2017. doi: 10.21037/atm.2017.03.42 View this article at: http://dx.doi.org/10.21037/atm.2017.03.42 The recent article by Schoellhammer et al., “Ultrasound- intensity ultrasound frequencies followed by quantification mediated gastrointestinal drug delivery” primarily of delivery of permeants (e.g., glucose, dextran, insulin). addresses practical limitations in drug delivery for medical Treated tissues showed enhanced transport. Similar management of inflammatory bowel disease (IBD), and findings were demonstrated in small and large bowel tissue presents pre-clinical data demonstrating that intra- for radiolabeled mesalamine and hydrocortisone. With 1 luminal, sub-ablative focused ultrasound (FUS), delivered minute of ultrasound treatment time, 3–5-fold improved via a trans-rectal transducer, can overcome some of these drug delivery was observed versus control. Additional limitations (1). The clinical application and benefit of such a ex vivo experiments utilizing variable FUS protocols to device is clear. -

Comprehensive Review on Herbal Toothpaste
Annals of R.S.C.B., ISSN:1583-6258, Vol. 25, Issue 4, 2021, Pages. 9509 - 9518 Received 05 March 2021; Accepted 01 April 2021. Comprehensive Review on Herbal Toothpaste Divya S1, Dr. J Suresh1*, Dr. S. Meenakshi 2 1. Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 2.Department of Prosthodontics, JSS Dental College, JSS Academy of Higher Education &Research, Mysuru-570015 Corresponding author Dr. J. Suresh Professor Department of Pharmacognosy, JSS College of Pharmacy, JSS Academy of Higher Education & Research, Mysuru-570015 Email: [email protected] Mobile No: 9480197611 ABSTRACT Herbal products for general as well as for oral health care have gained prominence around worldwide. People who aspire towards the use of herbal products often consider these products are relatively safer than products containing synthetic ingredients. Based on increased usage of herbal cosmetics we tried to make a comprehensive review on herbal toothpaste that helps to maintain a proper oral hygiene and free from periodontal disorder, reduce stain, gingivitis, calculus and caries. The present review gives basic information regarding antimicrobial potential of various herbs, formulationexcipients, that can be used in preparation of toothpaste. Key Words: Herbal toothpaste, Anti-microbial screening, Periodontal disorder, Gingivitis, calculus, Dental caries. INTRODUCTION In developing countries, the intensity of infections caused by certain pathogenic micro- organisms that may leads to mortality as well as morbidity in immune-suppressant patients [1]. Multiple abrasives, scent, green lead was used to remove the stain from teeth until mid- 19thcentury. In Medieval period rock salt, fine sand were the key ingredients used by Arabs for tooth cleaning.In the period 1950 AD, Dr. -

Chapter 1 Controlling Drug Delivery
chapter 1 Controlling drug delivery Overview In this chapter we will: & differentiate drug delivery systems according to their physical state & differentiate drug delivery systems according to their route of administration & differentiate drug delivery systems according to their type of drug release & discuss drug transport across epithelial barriers. Introduction KeyPoints & Continued developments in Pharmacotherapy can be defined as the treatment chemistry, molecular biology and prevention of illness and disease by means of and genomics support the drugs of chemical or biological origin. It ranks discovery and developments among the most important methods of medical of new drugs and new drug treatment, together with surgery, physical targets. & treatment, radiation and psychotherapy. There The drug delivery system are many success stories concerning the use of employed can control the pharmacological action of a drugs and vaccines in the treatment, prevention drug, influencing its and in some cases even eradication of diseases pharmacokinetic and (e.g. smallpox, which is currently the only subsequent therapeutic human infectious disease completely profile. eradicated). Although it is almost impossible to estimate the exact extent of the impact of pharmacotherapy on human health, there can be no doubt that pharmacotherapy, together with improved sanitation, better diet and better housing, has improved people’s health, life expectancy and quality of life. Tip Unprecedented developments in genomics Combinatorial chemistry is a way to and molecular biology today offer a plethora of build a variety of structurally related new drug targets. The use of modern chemical drug compounds rapidly and synthetic methods (such as combinatorial systematically. These are assembled chemistry) enables the syntheses of a large from a range of molecular entities number of new drug candidates in shorter times which are put together in different ‘ ’ than ever before. -

WO 2010/044736 Al
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date 22 April 2010 (22.04.2010) WO 2010/044736 Al (51) International Patent Classification: (74) Agents: Bratt, Henrik et al; Jarnvagsgatan 10 A, S-251 A61K 9/24 (2006.01) A61P 25/34 (2006.01) 10 Helsingborg (SE). (21) International Application Number: (81) Designated States (unless otherwise indicated, for every PCT/SE2009/05 1163 kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BR, BW, BY, BZ, (22) Date: International Filing CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, DO, 13 October 2009 (13.10.2009) DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, (25) Filing Language: English HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, (26) Publication Language: English ME, MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, (30) Priority Data: NO, NZ, OM, PE, PG, PH, PL, PT, RO, RS, RU, SC, SD, 0802 189-1 14 October 2008 (14.10.2008) SE SE, SG, SK, SL, SM, ST, SV, SY, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (71) Applicant (for all designated States except US): McNeil AB [SE/SE]; Box 941, S-25 1 09 Helsingborg (SE). (84) Designated States (unless otherwise indicated, for every kind of regional protection available): ARIPO (BW, GH, (72) Inventors; and GM, KE, LS, MW, MZ, NA, SD, SL, SZ, TZ, UG, ZM, (75) Inventors/Applicants (for US only): LINDELL, Katari- ZW), Eurasian (AM, AZ, BY, KG, KZ, MD, RU, TJ, na [SE/SE]; Skarhult 1325, S-241 93 Eslδv (SE). -

Review On: Sublingual Route for Systemic Drug Delivery
IAJPS 2018, 05 (01), 453-462 Himanshi Rathaur and G.Gnanarajan ISSN 2349-7750 CODEN [USA]: IAJPBB ISSN: 2349-7750 INDO AMERICAN JOURNAL OF PHARMACEUTICAL SCIENCES http://doi.org/10.5281/zenodo.1161209 Available online at: http://www.iajps.com Review Article REVIEW ON: SUBLINGUAL ROUTE FOR SYSTEMIC DRUG DELIVERY Himanshi Rathaur*1and G.Gnanarajan2 *1Shri Guru Ram Rai Institute of Technology and Sciences, Department of Pharmaceutics, Uttarakhand Technical University, Dehradun,248001, Uttarakhand, India 2Shri Guru Ram Rai Institute of Technology and Sciences, Department of Pharmaceutics, Faculty of Pharmacy, Shri Guru Ram Rai University, Dehradun, Uttarakhand, India. Abstract: Delivery of drug in the oral cavity through the oral mucosa is examined to be a promising alternative to the oral route. Sublingual means “under the tongue” which rapidly absorb the drug through the oral mucosa and enter into the systemic circulation. This route provides various advantages such as quick onset of action, patient compliance, hepatic first pass metabolism and increase bioavailability. Dysphagia is a common problem in pediatric, geriatric and psychiatric patients. In terms of permeability sublingual area of oral cavity is more permeable than buccal area which is in turn is more permeable than palatal area. Now a days most of the population need effective, faster and better relief within a short period of time. So, this route is the most appropriate route of administration and it rapidly dissolves in saliva. Many drugs like cardiovascular drugs, steroids, -
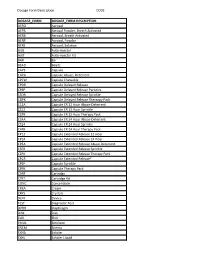
Dosage Form Description CODE
Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION AERO Aerosol AEPB Aerosol Powder, Breath Activated AERB Aerosol, Breath Activated AERP Aerosol, Powder AERS Aerosol, Solution AUIJ Auto-injector AJKT Auto-injector Kit BAR Bar BEAD Beads CAPS Capsule CAPA Capsule Abuse- Deterrent CPCW Capsule Chewable CPDR Capsule Delayed Release CPEP Capsule Delayed Release Particles CSDR Capsule Delayed Release Sprinkle CDPK Capsule Delayed Release Thereapy Pack C12A Capsule ER 12 Hour Abuse-Deterrent CS12 Capsule ER 12 Hour Sprinkle C2PK Capsule ER 12 Hour Therapy Pack C24A Capsule ER 24 Hour Abuse-Deterrent CS24 Capsule ER 24 Hour Sprinkle C4PK Capsule ER 24 Hour Therapy Pack CP12 Capsule Extended Release 12 Hour CP24 Capsule Extended Release 24 Hour CPEA Capsule Extended Release Abuse-Deterrent CSER Capsule Extended Release Sprinkle CEPK Capsule Extended Release Therapy Pack CPCR Capsule Extended Release* CPSP Capsule Sprinkle CPPK Capsule Therapy Pack CART Cartridge CTKT Cartridge Kit CONC Concentrate CREA Cream CRYS Crystals DEVI Device TEST Diagnostic Test DPRH Diaphragm DISK Disk ELIX Elixir EMUL Emulsion ENEM Enema EXHA Exhaler EXHL Exhaler Liquid Dosage Form Description CODE DOSAGE_FORM DOSAGE_FORM DESCRIPTION EXHP Exhaler Powder EXHS Exhaler Solution EXHU Exhaler Suspension FILM Film FLAK Flakes EXTR Fluid Extract FOAM Foam GAS Gas GEL Gel SOLG Gel Forming Solution GRAN Granules GREF Granules Effervescent GUM Gum IMPL Implant INHA Inhaler INJ Injectable INST Insert IUD Intrauterine Device JTAJ Jet-injector (Needleless) JTKT Jet-injector -

Formulation and Evaluation of Liposomal Drug Delivery System for Doxorubicin Hydrochloride
FORMULATION AND EVALUATION OF LIPOSOMAL DRUG DELIVERY SYSTEM FOR DOXORUBICIN HYDROCHLORIDE A dissertation submitted to THE TAMILNADU Dr. M.G.R.MEDICAL UNIVERSITY, CHENNAI. In partial fulfillment of the requirements for the award of degree of MASTER OF PHARMACY IN PHARMACEUTICS BY REG.NO: 26091390 Under the Guidance of Prof. S.P.SENTHIL, M.Pharm., ( Ph.D.,) OCTOBER -2011 THE ERODE COLLEGE OF PHARMACY AND RESEARCH INSTITUTE ERODE -638112, TAMILNADU DEDICATED TO My Beloved Family, Teachers & Friends CERTIFICATES The Erode College Of Pharmacy and Research Institute Prof.S.P.SENTHIL, M.Pharm.,( Ph.D.,) Department of pharmaceutics, Perundurai Main Road, Veppampalayam, Erode-638112, India. e-mail : [email protected] CERTIFICATE This is to certify that the investigation in this thesis entitled “FORMULATION AND EVALUATION OF LIPOSOMAL DRUG DELIVERY SYSTEM FOR DOXORUBICIN HYDROCHLORIDE” submitted to The Tamilnadu Dr. M.G.R. Medical University Chennai. For partial fulfillment of the award of degree of Master of pharmacy in Pharmaceutics was carried out by Reg. No: 26091390 in the department of pharmaceutics, The Erode College of pharmacy, Erode, under my guidance and supervision This work is original and has not been submitted in part or full to any other degree or diploma of this or any other university. Place: Erode Prof. S.P.SENTHIL, M.Pharm.,(Ph.D.,) Date: The Erode College Of Pharmacy and Research Institute Dr.V.Ganesan, M.Pharm., Ph.D., Professor and HOD of Pharmaceutics, Perundurai Main Road, Veppampalayam, Erode-638112, India. CERTIFICATE This is to certify that the investigation in this thesis entitled “FORMULATION AND EVALUATION OF LIPOSOMAL DRUG DELIVERY SYSTEM FOR DOXORUBICIN HYDROCHLORIDE ” submitted to the Tamil Nadu Dr. -

Medicinal Cannabis Dosage Forms in California an Overview of Cannabis Dosage Forms
Mica Gross President Sante Botanica / COO MDBioLogics Medicinal Cannabis Dosage Forms in California An Overview of Cannabis Dosage Forms Forms of dosage are the specific vehicles by which cannabis, or cannabinoids, are delivered into the body. Cannabis (drug-containing plant) —> The “form” in which THC and other cannabinoids Extracted Oil (drug substance) —> are administered determines how they moves and Delivery / Intake (drug product) works medicinally inside the body: ie the pharmacokinetics of cannabis. Both the plant material and the oils yielded through the extraction process influence the performance of the different forms of intake, which each have a particular set of benefits. An Overview of Cannabis Dosage Forms The medicinal effect experienced by the end- user is a function of the quality of the raw material – the cannabinoid and terpene profile of the cannabis – and of the derived oil yielded during extraction. The goal is to preserve the fidelity of the volatile medicinal compounds – such as the cannabinoids, terpenes, and flavonoids – throughout the entire chain of custody so that the therapeutic effect is intact and the dose is delivered in the most efficacious means possible. An Overview of Cannabis Dosage Forms Each form of intake is formulated to take advantage of the pharmacokinetic pathways as effectively as possible in delivering a therapeutic dose of cannabinoids (e.g. THC or CBD). An Overview of Cannabis Dosage Forms Important factors when considering each form of intake: • Bioavailability – the fraction of the administered -

Aqueous Emulsion Coating for Pharmaceutical Dosage Form
Europaisches Patentamt 0 347 024 3> European Patent Office J) Publication number: Dffice europeen des brevets 3) EUROPEAN PATENT APPLICATION © Application number: 89303290.4 © Int. CI.4: A61K 9/32 , A61K 9/36 , A61K 9/22 © Date of filing: 04.04.89 The title of the invention has been amended © Applicant: ALZA CORPORATION (Guidelines for Examination in the EPO, A-lll, 950 Page Mill Road P.O. Box 10950 7.3). Palo Alto California 94303-0802(US) © Inventor: Wong, Patrick S.-L. © Priority: 21.04.88 US 184478 2030 Cornell Street Palo Alto California 94306(US) © Date of publication of application: Inventor: Theeuwes, Felix 20.12.89 Bulletin 89/51 1634 Fallen Leaf Lane Los Altos California 94022(US) © Designated Contracting States: AT BE CH DE ES FR GB GR IT LI LU NL SE © Representative: Evans, David Charles et al F.J. CLEVELAND & COMPANY 40-43, Chancery Lane London, WC2A UQ(GB) © Aqueous emulsion coating for pharmaceutical dosage form. © A dosage form is disclosed comprising a cured emulsion coat that surrounds a drug. The emulsion comprises a lower alkyl acrylate-lower alkyl methacrylate copolymer and ethyl cellulose. The emulsion optionally comprises a hydrophilic poly- mer. eg < CM CO CL LU Xerox Copy Centre 1 EP 0 347 024 A2 2 AQUEOUS EMULSION FOR PHARMACEUTICAL DOSAGE FORM This invention pertains to a pharmaceutical final dosage form. dosage form comprising an aqueous emulsion coat It will be appreciated by those skilled in the and to an aqueous emulsion coat. drug dispensing art that if a coating is provided that is substantially free of organic solvents when coat- 5 ing drugs, drug granules, drug powders, drug dis- BACKGROUND OF THE INVENTION pensers, and the like, such a coating would have an immediate positive value and, concomitantly, represent an advancement in the drug coating art.