A Guide to Aerosol Delivery Devices for Respiratory Therapists 4Th Edition
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inhalation Devices: Various Forms of Administration for Therapeutic Optimization
vv ISSN: 2640-8082 DOI: https://dx.doi.org/10.17352/oja CLINICAL GROUP Renata Cristina de Angelo Calsaverini Leal* Review Article Santa Fé do Sul Foundation of Education and Culture, Brazil Inhalation Devices: Various forms Dates: Received: 31 May, 2017; Accepted: 26 June, of administration for Therapeutic 2017; Published: 27 June, 2017 *Corresponding author: Renata Cristina de Angelo Optimization Calsaverini Leal, Santa Fé do Sul Foundation of Education and Culture, Brazil, Tel: 55 (17) 3272-2769, E-mail: Keywords: Inhalation; Aerosol; Nebulizer Summary https://www.peertechz.com Introduction: Aerosol therapy consists of spraying liquid particles suspended for therapeutic purposes in the respiratory tract. With direct absorption and deposition at the lung level, avoiding side effects and presenting fast response time. Several factors infl uence the drug action, such as size, particle movement, ventilatory fl ow, pulmonary expansion, anatomy, respiratory mechanics and the nebulizer and patient interface. The therapeutic optimization depends on the type of nebulizer differentiating itself by the physical principle that generates the mist. Objectives: Check advantages and disadvantages of different inhalation devices. Methodology. This is a review of the PubMed database using descriptors: ultrasonic and jet nebulizer, aerosol deposition in the lung, metered dose inhaler and dry, inhaler therapy. Results: Different devices are mentioned in the literature: pneumatic and ultrasonic nebulizers (administering solutions), metered pressurized inhalers - pMDI used with or without expander chamber (administering suspensions) and dry powder inhalers - DPI (administering powder). Discussion and Conclusion: The US has advantages: quiet, does not require coordinating abilities, without propellant gases and quick nebulization with small amount of solution. Disadvantages: change in the active principle of thermosensitive drugs, deposition in the oropharynx and VAI of 2% of inhaled particles. -

Intravenous Therapy Procedure Manual
INTRAVENOUS THERAPY PROCEDURE MANUAL - 1 - LETTER OF ACCEPTANCE __________________________________________ hereby approves (Facility) the attached Reference Manual as of _____________________. (Date) The Intravenous Therapy Procedure Manual will be reviewed at least annually or more often when deemed appropriate. Revisions will be reviewed as they occur. Current copies of the Intravenous Therapy Procedure Manual shall be maintained at each appropriate nursing station. I have reviewed this manual and agree to its approval. __________________________ (Administrator) __________________________ (Director of Nursing) __________________________ (Medical Director) - 2 - TABLE OF CONTENTS TABLE OF CONTENTS INTRODUCTION A. Purpose 1 B. Local Standard of Practice 1 RESPONSIBILITIES A. Responsibilities: M Chest Pharmacy 1 B. Responsibilities: Administrator 1 C. Responsibilities: Director of Nursing Services (DON/DNS) 1 D. Skills Validation 2 AMENDMENTS GUIDELINES A. Resident Candidacy for IV Therapy 1 B. Excluded IV Medications and Therapies 1 C. Processing the IV Order 1 D. IV Solutions/Medications: Storage 2 E. IV Solutions/Medications: Handling 3 F. IV Solutions and Supplies: Destroying and Returning 4 G. IV Tubing 5 H. Peripheral IV Catheters and Needles 6 I. Central Venous Devices 7 J. Documentation and Monitoring 8 K. IV Medication Administration Times 9 L. Emergency IV Supplies 10 I TABLE OF CONTENTS PROTOCOLS A. IV Antibiotic 1 1. Purpose 2. Guidelines 3. Nursing Responsibilities B. IV Push 2 1. Purpose 2. Guidelines C. Anaphylaxis Allergic Reaction 4 1. Purpose 2. Guidelines 3. Nursing Responsibilities and Interventions 4. Signs and Symptoms of Anaphylaxis 5. Drugs Used to Treat Anaphylaxis 6. Physician Protocol PRACTICE GUIDELINES A. Purpose 1 B. Personnel 1 C. Competencies 1 D. -

Inhaled Steroids in Asthma a Patient's Guide
I I v v v v d v v Patient advice and liaison service (PALS) Actions r v If you have a compliment, complaint or It is sensible to ensure you make the r concern please contact our PALS team on following steps now you have a new r 020 7288 5551 or inhaler d [email protected] d d Pick up our guide on inhalers and If you need a large print, audio or spacers to complement this leaflet translated copy of this leaflet please Inhaled Steroids in Agree on a personalised asthma contact us on 020 7288 3182. We will try plan (this is done with your doctor or our best to meet your needs. Asthma nurse and usually written down for future reference) Remember to take your medicines A patient’s guide as advised Should your inhalers fail to relieve your symptoms, go straight to Accident and Emergency Need Help? Whittington Paediatric Asthma Nurse Tel: 020 7288 5527 Whittington Health Community Nurse Magdala Avenue Islington 020 3316 1950 (8am-6pm) London N19 5NF Haringey 020 8887 3301 (9am – 5pm) Phone: 020 7272 3070 www.whittington.nhs.uk Asthma UK 0300 222 5800 Date published: 25/09/2018 www.asthma.org.uk Review date: 25/09/2020 Ref: C&YP/Paed/ISA/03 © Whittington Health Please recycle Tel: 020 7272 3070 Asthma There are many types of preventer Side Effects Asthma is a common condition affecting inhaler. There are simple steroids like Parents worry about children and young the airway. Usually a trigger (such as dust beclomethasone, and then there are also adults taking inhaled steroids because of or pollen) irritates the airways which combined inhalers, called seretide or side effects they’ve heard about. -

Product Catalog POLICIES Ordering Options Payment Methods
Product Catalog POLICIES Ordering Options Payment Methods Three Easy Ways To Order Payment Portal and ACH Visit www.sunsethcs.com/paymentportal to pay online with Phone ACH or credit card. (877) 5-SUNSET (877-578-6738) Our customer service professionals are here to assist you Net 30-Day Payment Terms M-F 8:00 AM – 5:00 PM Central Time. For qualified companies. A 1.5% monthly finance charge will be assessed on all past due invoices. Fax 312-997-9985 — Accepted 24 hours a day. COD For non-qualified companies. Email [email protected] Credit Card We accept all major credit cards. A processing fee may apply. After 10 days of invoice date, a surcharge of 2.5% will be applied. Shipping Customer Service Drop shipping available — ask your sales rep Returns about sending your order to multiple locations. Call us for a return authorization number. There is no restocking charge on orders returned within 20 days of UPS invoice. Orders returned after 20 days may be assessed a Whenever possible, orders ship via UPS. If another carrier restocking fee of 15%. No returns after 90 days. is desired, please contact your sales rep. (877) 5-SUNSET (877-578-6738) All orders are packed in the smallest boxes available and Central Distribution Center Western Distribution Center combined with other products on the order to reduce Sunset Healthcare Solutions Sunset Healthcare Solutions shipping costs. 279 Madsen Dr Ste 101 2450 Statham Blvd Bloomingdale, IL 60108 Oxnard, CA 93033 All products ship prepaid and are added to the invoice. Problems All products ship from Bloomingdale, IL or Oxnard, CA. -

The Rapid Response Team Jimmy Phillips: Catawba Valley Medical Center Jessie Miller: Martin General Hospital
Panelists The Rapid Response Team Jimmy Phillips: Catawba Valley Medical Center Jessie Miller: Martin General Hospital Lawson Millner: Forsyth Medical Center A Panel Discussion Jhaymie Cappiello: Duke University Hospital Reason for RRT initiation Institute for Healthcare Improvement 2004 •66 % of patients showed abnormal signs & symptoms within 6 hours of arrest & the MD was notified in 25 % of those cases (Franklin & Mathew, 1994). 1. Delivery of Reliable, Evidence-Based Care for Acute Myocardial Infarction… • 2. Prevention of Adverse Drug Events (ADEs) 50% reduction in non-ICU arrests (Buist, BMJ 02) 3. Prevention of Central Line Infections •Reduction in arrest prior to ICU transfer (4 % v 30 %) (Goldhill, Anest 99) 4. Prevention of Surgical Site Infections •Reduced post-operative emergency ICU transfers (44%) and deaths (37%) 5. Prevention of Ventilator-Associated Pneumonia (Bellomo, CCM 04) 6. Deployment of a Rapid Response Team – early recognition and treatment of clinical deterioration can decrease the rate of cardioplulmonary arrests outside the critical care setting and unplanned intensive care admissions – The goal: To prevent deaths in patients who are failing outside intensive care settings. IHI Recommendations for an RRT 1. What Is the Role of the Rapid Response Team 2. Determine the Team Structure 3. Provide Education and Training Where Are We Now? 4. Establish Criteria for Calling the RRT 5. Mechanism for Calling the RRT 6. Communication and Documentation 7. Establish Feedback mechanisms and Measure Effectiveness Catawba -

Absorbine Veterinary Liniment for Horses
Doc# 03.287 Ver. 11 SAFETY DATA SHEET ABSORBINE® VETERINARY LINIMENT SECTION 1 - PRODUCT AND COMPANY IDENTIFICATION 1.1 Trade Name (as labeled): Absorbine® Veterinary Liniment Synonyms: N/A CAS No: Mixture 1.2 Product Use: Soothes sore muscles and stiff joints 1.3 Company Name: W.F. Young Company Address: 302 Benton Dr Company Address Cont: East Longmeadow, MA 01028 Business Phone: ( 413) 526-9999 Website: www.wfyoung.com 1.4 Emergency Telephone Number: (413) 526-9999 Date of Current Revision: January 17, 2017 Date of Last Revision: August 7, 2015 SECTION 2 - HAZARD IDENTIFICATION EMERGENCY OVERVIEW: This product is a green thin liquid with an acetone odor. Health Hazards: May cause skin, eye, and respiratory system irritation. Flammabilit Hazards: This product is a flammable liquid with a flash point over 20°F (-6. 7°C). Reactivit Hazards: None. Environmental Hazards: The environmental effectsof this product have not been investigated, however release may cause long term adverse environmental effects. US DOT Symbols: EU and GHS Symbols: Signal Word: Danger! 2.1 CLASSIFICATION OF SUBSTANCE OR MIXTURE IN ACCORDANCE WITH 29 CFR 1200 (OSHA HCSl AND THE EUROPEAN UNION DIRECTIVES: This product does meet the definition of a hazardous substance or preparation as defined by 29 CFR 1910. 1200 or the European Union Council Directives 67 /548/EEC, 1999/45/EC, 1272/2008/EC and subsequent Directives. EU HAZARD CLASSIFICATIONOF INGREDIENTS PER DIRECTIVE 1272/2008/EC: IndexNumber: EC# 201-939-0 This substance is not classified in the AnnexVI of Directive 67/548/EEC EC# 200-662-2 This substance is classified in the AnnexVI of Directive 67/548/EEC Index# 606-001-00-8 Substances not listed either individually or in group entries must be self classified. -

Priority for Everyone, and Even More So for Those with Chronic Illnesses
Mouth Care: Patient/Caregiver Training Hospice of Cincinnati Mouth Care is a high priority for everyone, and even more so for those with chronic illnesses. Proper mouth care has many benefits including: 1. Increasing appetite 2. Maintaining comfort 3. Clearer speech 4. Easier swallowing 5. Preventing sores and infection. General tips: Mouth care should be performed a minimum of twice daily and more frequently for dry mouth, if breathing through the mouth, or if you are not eating. Mouth care should always be performed after using an inhaler/nebulizer to prevent thrush. Thrush is slightly raised white patches or a rash typically seen on the tongue or inner cheeks. There may be pain with swallowing. Contact your hospice nurse immediately if you suspect thrush. Dentures may no longer fit well. You may consider using them only when eating or when visiting with friends and family. Questions or concerns? Call a hospice nurse at 513-891-7700. Training: Mouth Care. ©2017 Hospice of Cincinnati Hospice of Cincinnati Relieving dry mouth: 1. Increase frequency of mouth care. 2. Encourage sucking on candy, ice chips, popsicles or taking small sips of water to increase saliva. 3. Use of a mouthwash can increase dryness. Talk to your nurse about a proper mouth moisturizer/artificial saliva product. For those with difficulty swallowing: • Raise the head of the bed and support the head with pillows, turn head to one side. • Cover the upper body with a towel to keep the area clean and dry. • Remove dentures prior to mouth care and brush separately. • Use a toothette, which is a sponge-tipped oral swab, to clean the mouth and teeth using a small amount of toothpaste. -

An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: a Review
Muthadi Radhika Reddy /J. Pharm. Sci. & Res. Vol. 12(7), 2020, 925-940 An Introduction to Fast Dissolving Oral Thin Film Drug Delivery Systems: A Review Muthadi Radhika Reddy1* 1School of pharmacy, Gurunanak Institute of Technical Campus, Hyderabad, Telangana, India and Department of Pharmacy, Gandhi Institute of Technology and Management University, Vizag, Andhra Pradesh, India INTRODUCTION 2. Useful in situations where rapid onset of action Fast dissolving drug delivery systems were first developed required such as in motion sickness, allergic attack, in the late 1970s as an alternative to conventional dosage coughing or asthma forms. These systems consist of solid dosage forms that 3. Has wide range of applications in pharmaceuticals, Rx disintegrate and dissolve quickly in the oral cavity without Prescriptions and OTC medications for treating pain, the need of water [1]. Fast dissolving drug delivery cough/cold, gastro-esophageal reflux disease,erectile systems include orally disintegrating tablets (ODTs) and dysfunction, sleep disorders, dietary supplements, etc oral thin films (OTFs). The Centre for Drug Evaluation [4] and Research (CDER) defines ODTs as,“a solid dosage 4. No water is required for the administration and hence form containing medicinal substances which disintegrates suitable during travelling rapidly, usually within a matter of seconds, when placed 5. Some drugs are absorbed from the mouth, pharynx upon the tongue” [2]. USFDA defines OTFs as, “a thin, and esophagus as the saliva passes down into the flexible, non-friable polymeric film strip containing one or stomach, enhancing bioavailability of drugs more dispersed active pharmaceutical ingredients which is 6. May offer improved bioavailability for poorly water intended to be placed on the tongue for rapid soluble drugs by offering large surface area as it disintegration or dissolution in the saliva prior to disintegrates and dissolves rapidly swallowing for delivery into the gastrointestinal tract” [3]. -

Injection Technique 1: Administering Drugs Via the Intramuscular Route
Copyright EMAP Publishing 2018 This article is not for distribution except for journal club use Clinical Practice Keywords Intramuscular injection/ Medicine administration/Absorption Practical procedures This article has been Injection technique double-blind peer reviewed Injection technique 1: administering drugs via the intramuscular route rugs administered by the intra- concerns that nurses are still performing Author Eileen Shepherd is clinical editor muscular (IM) route are depos- outdated and ritualistic practice relating to at Nursing Times. ited into vascular muscle site selection, aspirating back on the syringe Dtissue, which allows for rapid (Greenway, 2014) and skin cleansing. Abstract The intramuscular route allows absorption into the circulation (Dough- for rapid absorption of drugs into the erty and Lister, 2015; Ogston-Tuck, 2014). Site selection circulation. Using the correct injection Complications of poorly performed IM Four muscle sites are recommended for IM technique and selecting the correct site injection include: administration: will minimise the risk of complications. l Pain – strategies to reduce this are l Vastus lateris; outlined in Box 1; l Rectus femoris Citation Shepherd E (2018) Injection l Bleeding; l Deltoid; technique 1: administering drugs via l Abscess formation; l Ventrogluteal (Fig 1, Table 1). the intramuscular route. Nursing Times l Cellulitis; Traditionally the dorsogluteal (DG) [online]; 114: 8, 23-25. l Muscle fibrosis; muscle was used for IM injections but this l Injuries to nerves and blood vessels muscle is in close proximity to a major (Small, 2004); blood vessel and nerves, with sciatic nerve l Inadvertent intravenous (IV) access. injury a recognised complication (Small, These complications can be avoided if 2004). -

Respiratory Therapy
Respiratory Therapy rio.edu The University of Rio Grande/Rio Grande Community treatment, management, control, diagnostic evaluation, College, in conjunction with Buckeye Hills Career Center, education, and care of patients with deficiencies and offers an allied health program in Respiratory abnormalities of the cardiopulmonary system, as well as on Therapy. The Respiratory Therapy program curriculum the prevention of the development of these deficiencies. includes courses in allied health sciences, math, chemistry, Critical thinking, patient and environment assessment skills, pharmacology and the professional courses in the respiratory Respiratory therapists work with sophisticated medical therapy field. Upon completion of the curriculum, the equipment such as mechanical ventilators that breathe for student will be granted an Associate of Applied Science people who can’t breathe on their own, and also work with degree in Respiratory Therapy from the University of Rio other devices that require a knowledge and ability to apply Grande/Rio Grande Community College. The graduate will high-tech devices in the care and treatment of patients. be eligible to apply to the National Board for Respiratory Respiratory therapists assess patients to ensure the treatments Care for his/her certification and registry exams. are working properly, and how to make the changes A respiratory therapist, also known as a respiratory care necessary to arrive at the best outcome for the patient. practitioner, evaluates, treats, and cares for patients with Approximately 81% of respiratory jobs are in hospitals, breathing or other cardiopulmonary disorders. Respiratory mainly in departments of respiratory care; anesthesiology; therapists, practicing under physician direction, assume or pulmonary medicine. Most of the remaining jobs were in primary responsibility for all respiratory care treatments and offices of physicians or other health practitioners, consumer- diagnostic procedures. -
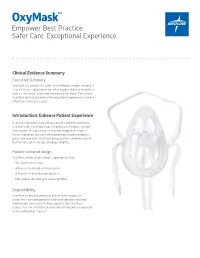
Oxymask™ Empower Best Practice
OxyMask™ Empower Best Practice. Safer Care. Exceptional Experience. Clinical Evidence Summary Executive Summary OxyMask is a solution for safer, more efficient oxygen delivery. It is an all-in-one replacement for other oxygen delivery modalities, such as the venturi mask and non-rebreather mask. The unique OxyMask technology helps enhance patient experience, increase efficiency, and reduce costs. Introduction: Enhance Patient Experience In an ever-competitive healthcare market, patient experience is at the forefront of advances in healthcare. Patients treated with oxygen therapy may feel they are trapped or helpless.1 Patient-centered solutions are increasingly recommended in policy and research.2 OxyMask brings patient-centered care to fruition through its design and dependability. Patient-centered design OxyMask utilizes a light-weight, open design that: • Sits lightly on the face • Allows unrestricted communication • Allows for oral medication delivery • May reduce the feeling of claustrophobia Dependability OxyMask is clinically proven to deliver more oxygen at lower flow rates compared to traditional delivery methods.3 Additionally, laboratory testing suggests that OxyMask reduces the risk of carbon dioxide rebreathing when compared to non-rebreather masks.4,5 OxyMask™ OxyMask Delivers Oxygen More Efficiently OxyMask May Reduce the Risk of Carbon Than a Venturi Mask3 Dioxide Rebreathing Compared to a Beecroft JM, Hanly PJ. Comparison of the OxyMask and Venturi mask in the Non-Rebreather Mask4 delivery of supplemental oxygen: Pilot study in oxygen-dependent patients. Can Respir J. 2006;13(5):247-252. Lamb K, Piper D. Southmedic OxyMaskTM compared with the Hudson RCI® Non-Rebreather Mask™: Safety and performance comparison. Can J Resp Ther. -

Inhalation Solution, USP for Oral Inhalation Use What Is Tobramycin
PATIENT INFORMATION TOBRAMYCIN (TOE-BRA-MYE-SIN) Inhalation Solution, USP for oral inhalation use What is Tobramycin Inhalation Solution? Tobramycin inhalation solution is a prescription medicine that is used to treat people with cystic fibrosis who have a bacterial infection called Pseudomonas aeruginosa. Tobramycin inhalation solution contains an antibacterial medicine called tobramycin (an aminoglycoside). It is not known if tobramycin inhalation solution is safe and effective: in children under 6 years of age in people who have an FEV1 less than 25% or greater than 75% predicted in people who are colonized with a bacterium called Burkholderia cepacia Do not take tobramycin inhalation solution if you are allergic to tobramycin, any of the ingredients in tobramycin inhalation solution, or to any other aminoglycoside antibacterial. See the end of this Patient Information for a complete list of ingredients in tobramycin inhalation solution. Before you take tobramycin inhalation solution, tell your healthcare provider about all of your medical conditions, including if you: have or have had hearing problems (including noises in your ears such as ringing or hissing) have dizziness have or have had kidney problems have or have had problems with muscle weakness such as myasthenia gravis or Parkinson’s disease have or have had breathing problems such as wheezing, coughing, or chest tightness are pregnant or plan to become pregnant. Tobramycin inhalation solution is in a class of drugs that can harm your unborn baby. are breastfeeding or plan to breastfeed. It is not known if tobramycin passes into your breast milk. are receiving aminoglycoside therapy by injection or through a vein (intravenous) while taking tobramycin inhalation solution.