Chen-J.-2008.Pdf
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

And Β-Keratins on Developing Chicken Skin Integuments: Functional Interaction and Evolutionary Perspectives
Topographical mapping of α- and β-keratins on developing chicken skin integuments: Functional interaction and evolutionary perspectives Ping Wua,1, Chen Siang Ngb,1, Jie Yana,c, Yung-Chih Laia,d,e, Chih-Kuan Chenb,f, Yu-Ting Laib, Siao-Man Wub, Jiun-Jie Chenb, Weiqi Luoa, Randall B. Widelitza, Wen-Hsiung Lib,g,2, and Cheng-Ming Chuonga,d,e,h,2 aDepartment of Pathology, Keck School of Medicine, University of Southern California, Los Angeles, CA 90033; bBiodiversity Research Center, Academia Sinica, Taipei 11529, Taiwan; cJiangsu Key Laboratory for Biodiversity and Biotechnology, College of Life Sciences, Nanjing Normal University, Nanjing 210023, China; dResearch Center for Developmental Biology and Regenerative Medicine, National Taiwan University, Taipei 10041, Taiwan; eIntegrative Stem Cell Center, China Medical University, Taichung 40447, Taiwan; fInstitute of Ecology and Evolutionary Biology, National Taiwan University, Taipei 10617, Taiwan; gDepartment of Ecology and Evolution, University of Chicago, Chicago, IL 60637; and hCenter for the Integrative and Evolutionary Galliform Genomics, National Chung Hsing University, Taichung 40227, Taiwan Contributed by Wen-Hsiung Li, October 19, 2015 (sent for review July 19, 2015; reviewed by Scott V. Edwards and Roger H. Sawyer) Avian integumentary organs include feathers, scales, claws, and innovations of feather development genes predate the origin of beaks. They cover the body surface and play various functions to feathers, suggesting that the avian dinosaur ancestor already had help adapt birds to diverse environments. These keratinized struc- the nonkeratin protein-coding toolkit for making feathers (12). α tures are mainly composed of corneous materials made of -keratins, While fewer new genes have been found in bird genomes (13) β which exist in all vertebrates, and -keratins, which only exist in birds and the α-keratin gene family has shrunk in birds relative to and reptiles. -

Universidade Estadual De Campinas Instituto De Biologia
UNIVERSIDADE ESTADUAL DE CAMPINAS INSTITUTO DE BIOLOGIA VERÔNICA APARECIDA MONTEIRO SAIA CEREDA O PROTEOMA DO CORPO CALOSO DA ESQUIZOFRENIA THE PROTEOME OF THE CORPUS CALLOSUM IN SCHIZOPHRENIA CAMPINAS 2016 1 VERÔNICA APARECIDA MONTEIRO SAIA CEREDA O PROTEOMA DO CORPO CALOSO DA ESQUIZOFRENIA THE PROTEOME OF THE CORPUS CALLOSUM IN SCHIZOPHRENIA Dissertação apresentada ao Instituto de Biologia da Universidade Estadual de Campinas como parte dos requisitos exigidos para a obtenção do Título de Mestra em Biologia Funcional e Molecular na área de concentração de Bioquímica. Dissertation presented to the Institute of Biology of the University of Campinas in partial fulfillment of the requirements for the degree of Master in Functional and Molecular Biology, in the area of Biochemistry. ESTE ARQUIVO DIGITAL CORRESPONDE À VERSÃO FINAL DA DISSERTAÇÃO DEFENDIDA PELA ALUNA VERÔNICA APARECIDA MONTEIRO SAIA CEREDA E ORIENTADA PELO DANIEL MARTINS-DE-SOUZA. Orientador: Daniel Martins-de-Souza CAMPINAS 2016 2 Agência(s) de fomento e nº(s) de processo(s): CNPq, 151787/2F2014-0 Ficha catalográfica Universidade Estadual de Campinas Biblioteca do Instituto de Biologia Mara Janaina de Oliveira - CRB 8/6972 Saia-Cereda, Verônica Aparecida Monteiro, 1988- Sa21p O proteoma do corpo caloso da esquizofrenia / Verônica Aparecida Monteiro Saia Cereda. – Campinas, SP : [s.n.], 2016. Orientador: Daniel Martins de Souza. Dissertação (mestrado) – Universidade Estadual de Campinas, Instituto de Biologia. 1. Esquizofrenia. 2. Espectrometria de massas. 3. Corpo caloso. -

Downregulation of Salivary Proteins, Protective Against Dental Caries, in Type 1 Diabetes
proteomes Article Downregulation of Salivary Proteins, Protective against Dental Caries, in Type 1 Diabetes Eftychia Pappa 1,* , Konstantinos Vougas 2, Jerome Zoidakis 2 , William Papaioannou 3, Christos Rahiotis 1 and Heleni Vastardis 4 1 Department of Operative Dentistry, School of Dentistry, National and Kapodistrian University of Athens, 11527 Athens, Greece; [email protected] 2 Proteomics Laboratory, Biomedical Research Foundation Academy of Athens, 11527 Athens, Greece; [email protected] (K.V.); [email protected] (J.Z.) 3 Department of Preventive and Community Dentistry, School of Dentistry, National and Kapodistrian University of Athens, 11527 Athens, Greece; [email protected] 4 Department of Orthodontics, School of Dentistry, National and Kapodistrian University of Athens, 11527 Athens, Greece; [email protected] * Correspondence: effi[email protected] Abstract: Saliva, an essential oral secretion involved in protecting the oral cavity’s hard and soft tissues, is readily available and straightforward to collect. Recent studies have analyzed the sali- vary proteome in children and adolescents with extensive carious lesions to identify diagnostic and prognostic biomarkers. The current study aimed to investigate saliva’s diagnostic ability through proteomics to detect the potential differential expression of proteins specific for the occurrence of carious lesions. For this study, we performed bioinformatics and functional analysis of proteomic datasets, previously examined by our group, from samples of adolescents with regulated and unreg- ulated type 1 diabetes, as they compare with healthy controls. Among the differentially expressed Citation: Pappa, E.; Vougas, K.; proteins relevant to caries pathology, alpha-amylase 2B, beta-defensin 4A, BPI fold containing family Zoidakis, J.; Papaioannou, W.; Rahiotis, C.; Vastardis, H. -

Diabetes Induced Alterations in Murine Vitreous Proteome Are Mitigated by IL-6 Trans-Signaling Inhibition
Retina Diabetes Induced Alterations in Murine Vitreous Proteome Are Mitigated by IL-6 Trans-Signaling Inhibition Rebekah Robinson,1 Hannah Youngblood,2 Hersha Iyer,1 Justin Bloom,1 Tae Jin Lee,1 Luke Chang,3 Zachary Lukowski,3 Wenbo Zhi,1 Ashok Sharma,1,3–5 and Shruti Sharma1,3,5 1Center for Biotechnology and Genomic Medicine, Augusta University, Augusta, Georgia, United States 2Department of Cellular Biology and Anatomy, Augusta University, Augusta, Georgia, United States 3Department of Ophthalmology, Augusta University, Augusta, Georgia, United States 4Department of Population Health Sciences, Augusta University, Augusta, Georgia, United States 5Culver Vision Discovery Institute, Augusta University, Augusta, Georgia, United States Correspondence: Shruti Sharma, PURPOSE. Diabetic retinopathy (DR) is a microvascular complication caused by prolonged Center for Biotechnology and hyperglycemia and characterized by leaky retinal vasculature and ischemia-induced Genomic Medicine, Medical College angiogenesis. Vitreous humor is a gel-like biofluid in the posterior segment of the eye of Georgia, Augusta University, 1460 between the lens and the retina. Disease-related changes are observed in the biochem- Laney Walker Blvd, CAII 4139, ical constituents of the vitreous, including proteins and macromolecules. Recently, we Augusta, GA 30912, USA; [email protected]. found that IL-6 trans-signaling plays a significant role in the vascular leakage and retinal pathology associated with DR. Therefore, in this study, comprehensive proteomic profil- Received: May 18, 2020 ing of the murine vitreous was performed to identify diabetes-induced alterations and to Accepted: August 5, 2020 determine effects of IL-6 trans-signaling inhibition on these changes. Published: September 1, 2020 METHODS. Vitreous samples from mice were collected by evisceration, and proteomic Citation: Robinson R, Youngblood H, Iyer H, et al. -

Proteomic Expression Profile in Human Temporomandibular Joint
diagnostics Article Proteomic Expression Profile in Human Temporomandibular Joint Dysfunction Andrea Duarte Doetzer 1,*, Roberto Hirochi Herai 1 , Marília Afonso Rabelo Buzalaf 2 and Paula Cristina Trevilatto 1 1 Graduate Program in Health Sciences, School of Medicine, Pontifícia Universidade Católica do Paraná (PUCPR), Curitiba 80215-901, Brazil; [email protected] (R.H.H.); [email protected] (P.C.T.) 2 Department of Biological Sciences, Bauru School of Dentistry, University of São Paulo, Bauru 17012-901, Brazil; [email protected] * Correspondence: [email protected]; Tel.: +55-41-991-864-747 Abstract: Temporomandibular joint dysfunction (TMD) is a multifactorial condition that impairs human’s health and quality of life. Its etiology is still a challenge due to its complex development and the great number of different conditions it comprises. One of the most common forms of TMD is anterior disc displacement without reduction (DDWoR) and other TMDs with distinct origins are condylar hyperplasia (CH) and mandibular dislocation (MD). Thus, the aim of this study is to identify the protein expression profile of synovial fluid and the temporomandibular joint disc of patients diagnosed with DDWoR, CH and MD. Synovial fluid and a fraction of the temporomandibular joint disc were collected from nine patients diagnosed with DDWoR (n = 3), CH (n = 4) and MD (n = 2). Samples were subjected to label-free nLC-MS/MS for proteomic data extraction, and then bioinformatics analysis were conducted for protein identification and functional annotation. The three Citation: Doetzer, A.D.; Herai, R.H.; TMD conditions showed different protein expression profiles, and novel proteins were identified Buzalaf, M.A.R.; Trevilatto, P.C. -

Onset of Taste Bud Cell Renewal Starts at Birth and Coincides with a Shift In
RESEARCH ARTICLE Onset of taste bud cell renewal starts at birth and coincides with a shift in SHH function Erin J Golden1,2, Eric D Larson2,3, Lauren A Shechtman1,2, G Devon Trahan4, Dany Gaillard1,2, Timothy J Fellin1,2, Jennifer K Scott1,2, Kenneth L Jones4, Linda A Barlow1,2* 1Department of Cell & Developmental Biology, University of Colorado Anschutz Medical Campus, Aurora, United States; 2The Rocky Mountain Taste and Smell Center, University of Colorado Anschutz Medical Campus, Aurora, United States; 3Department of Otolaryngology, University of Colorado Anschutz Medical Campus, Aurora, United States; 4Department of Pediatrics, Section of Hematology, Oncology, and Bone Marrow Transplant, University of Colorado Anschutz Medical Campus, Aurora, United States Abstract Embryonic taste bud primordia are specified as taste placodes on the tongue surface and differentiate into the first taste receptor cells (TRCs) at birth. Throughout adult life, TRCs are continually regenerated from epithelial progenitors. Sonic hedgehog (SHH) signaling regulates TRC development and renewal, repressing taste fate embryonically, but promoting TRC differentiation in adults. Here, using mouse models, we show TRC renewal initiates at birth and coincides with onset of SHHs pro-taste function. Using transcriptional profiling to explore molecular regulators of renewal, we identified Foxa1 and Foxa2 as potential SHH target genes in lingual progenitors at birth and show that SHH overexpression in vivo alters FoxA1 and FoxA2 expression relevant to taste buds. We further bioinformatically identify genes relevant to cell adhesion and cell *For correspondence: locomotion likely regulated by FOXA1;FOXA2 and show that expression of these candidates is also LINDA.BARLOW@CUANSCHUTZ. altered by forced SHH expression. -

Transcriptome Profiling and Differential Gene Expression In
G C A T T A C G G C A T genes Article Transcriptome Profiling and Differential Gene Expression in Canine Microdissected Anagen and Telogen Hair Follicles and Interfollicular Epidermis Dominique J. Wiener 1,* ,Kátia R. Groch 1 , Magdalena A.T. Brunner 2,3, Tosso Leeb 2,3 , Vidhya Jagannathan 2 and Monika M. Welle 3,4 1 Department of Veterinary Pathobiology, College of Veterinary Medicine & Biomedical Science, Texas A&M University, College Station, TX 77843, USA; [email protected] 2 Institute of Genetics, Vetsuisse Faculty, University of Bern, 3012 Bern, Switzerland; [email protected] (M.A.T.B.); [email protected] (T.L.); [email protected] (V.J.) 3 Dermfocus, Vetsuisse Faculty, University Hospital of Bern, 3010 Bern, Switzerland; [email protected] 4 Institute of Animal Pathology, Vetsuisse Faculty, University of Bern, 3012 Bern, Switzerland * Correspondence: [email protected]; Tel.: +1-979-862-1568 Received: 30 June 2020; Accepted: 3 August 2020; Published: 4 August 2020 Abstract: The transcriptome profile and differential gene expression in telogen and late anagen microdissected hair follicles and the interfollicular epidermis of healthy dogs was investigated by using RNAseq. The genes with the highest expression levels in each group were identified and genes known from studies in other species to be associated with structure and function of hair follicles and epidermis were evaluated. Transcriptome profiling revealed that late anagen follicles expressed mainly keratins and telogen follicles expressed GSN and KRT15. The interfollicular epidermis expressed predominately genes encoding for proteins associated with differentiation. All sample groups express genes encoding for proteins involved in cellular growth and signal transduction. -

Types I and II Keratin Intermediate Filaments
Downloaded from http://cshperspectives.cshlp.org/ on October 10, 2021 - Published by Cold Spring Harbor Laboratory Press Types I and II Keratin Intermediate Filaments Justin T. Jacob,1 Pierre A. Coulombe,1,2 Raymond Kwan,3 and M. Bishr Omary3,4 1Department of Biochemistry and Molecular Biology, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland 21205 2Departments of Biological Chemistry, Dermatology, and Oncology, School of Medicine, and Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University, Baltimore, Maryland 21205 3Departments of Molecular & Integrative Physiologyand Medicine, Universityof Michigan, Ann Arbor, Michigan 48109 4VA Ann Arbor Health Care System, Ann Arbor, Michigan 48105 Correspondence: [email protected] SUMMARY Keratins—types I and II—are the intermediate-filament-forming proteins expressed in epithe- lial cells. They are encoded by 54 evolutionarily conserved genes (28 type I, 26 type II) and regulated in a pairwise and tissue type–, differentiation-, and context-dependent manner. Here, we review how keratins serve multiple homeostatic and stress-triggered mechanical and nonmechanical functions, including maintenance of cellular integrity, regulation of cell growth and migration, and protection from apoptosis. These functions are tightly regulated by posttranslational modifications and keratin-associated proteins. Genetically determined alterations in keratin-coding sequences underlie highly penetrant and rare disorders whose pathophysiology reflects cell fragility or altered -

Mice Expressing a Mutant Krt75 (K6hf) Allele Develop Hair and Nail
ORIGINAL ARTICLE Mice Expressing a Mutant Krt75 (K6hf ) Allele Develop Hair and Nail Defects Resembling Pachyonychia Congenita Jiang Chen1, Karin Jaeger2,3, Zhining Den4, Peter J. Koch1,4,5, John P. Sundberg2 and Dennis R. Roop1,4,5 KRT75 (formerly known as K6hf) is one of the isoforms of the keratin 6 (KRT6) family located within the type II cytokeratin gene cluster on chromosome 12 of humans and chromosome 15 of mice. KRT75 is expressed in the companion layer and upper germinative matrix region of the hair follicle, the medulla of the hair shaft, and in epithelia of the nail bed. Dominant mutations in members of the KRT6 family, such as in KRT6A and KRT6B cause pachyonychia congenita (PC) -1 and -2, respectively. To determine the function of KRT75 in skin appendages, we introduced a dominant mutation into a highly conserved residue in the helix initiation peptide of Krt75. Mice expressing this mutant form of Krt75 developed hair and nail defects resembling PC. This mouse model provides in vivo evidence for the critical roles played by Krt75 in maintaining hair shaft and nail integrity. Furthermore, the phenotypes observed in our mutant Krt75 mice suggest that KRT75 may be a candidate gene for screening PC patients who do not exhibit obvious mutations in KRT6A, KRT6B, KRT16,orKRT17, especially those with extensive hair involvement. Journal of Investigative Dermatology (2008) 128, 270–279; doi:10.1038/sj.jid.5701038; published online 13 September 2007 INTRODUCTION the skin, hair follicles, and nails. However, each member of The keratin 6 (KRT6) cluster consists of several genes that the KRT6 gene family shows a cell- and tissue-type-specific encode intermediate filament (IF) proteins belonging to the expression pattern. -

Mapping Transmembrane Binding Partners for E-Cadherin Ectodomains
SUPPLEMENTARY INFORMATION TITLE: Mapping transmembrane binding partners for E-cadherin ectodomains. AUTHORS: Omer Shafraz 1, Bin Xie 2, Soichiro Yamada 1, Sanjeevi Sivasankar 1, 2, * AFFILIATION: 1 Department of Biomedical Engineering, 2 Biophysics Graduate Group, University of California, Davis, CA 95616. *CORRESPONDING AUTHOR: Sanjeevi Sivasankar, Tel: (530)-754-0840, Email: [email protected] Figure S1: Western blots a. EC-BioID, WT and Ecad-KO cell lysates stained for Ecad and tubulin. b. HRP-streptavidin staining of biotinylated proteins eluted from streptavidin coated magnetic beads incubated with cell lysates of EC-BioID with (+) and without (-) exogenous biotin. c. C-BioID, WT and Ecad-KO cell lysates stained for Ecad and tubulin. d. HRP-streptavidin staining of biotinylated proteins eluted from streptavidin coated magnetic beads incubated with cell lysates of C-BioID with (+) and without (-) exogenous biotin. (+) Biotin (-) Biotin Sample 1 Sample 2 Sample 3 Sample 4 Sample 1 Sample 2 Sample 3 Sample 4 Percent Percent Percent Percent Percent Percent Percent Percent Gene ID Coverage Coverage Coverage Coverage Coverage Coverage Coverage Coverage CDH1 29.6 31.4 41.1 36.5 10.8 6.7 28.8 29.1 DSG2 26 14.6 45 37 0.8 1.9 1.6 18.7 CXADR 30.2 26.2 32.7 27.1 0.0 0.0 0.0 6.9 EFNB1 24.3 30.6 24 30.3 0.0 0.0 0.0 0.0 ITGA2 16.5 22.2 30.1 33.4 1.1 1.1 5.2 7.2 CDH3 21.8 9.7 20.6 25.3 1.3 1.3 0.0 0.0 ITGB1 11.8 16.7 23.9 20.3 0.0 2.9 8.5 5.8 DSC3 9.7 7.5 11.5 13.3 0.0 0.0 2.6 0.0 EPHA2 23.2 31.6 31.6 30.5 0.8 0.0 0.0 5.7 ITGB4 21.8 27.8 33.1 30.7 0.0 1.2 3.9 4.4 ITGB3 23.5 22.2 26.8 24.7 0.0 0.0 5.2 9.1 CDH6 22.8 18.1 28.6 24.3 0.0 0.0 0.0 9.1 CDH17 8.8 12.4 20.7 18.4 0.0 0.0 0.0 0.0 ITGB6 12.7 10.4 14 17.1 0.0 0.0 0.0 1.7 EPHB4 11.4 8.1 14.2 16.3 0.0 0.0 0.0 0.0 ITGB8 5 10 15 17.6 0.0 0.0 0.0 0.0 ITGB5 6.2 9.5 15.2 13.8 0.0 0.0 0.0 0.0 EPHB2 8.5 4.8 9.8 12.1 0.0 0.0 0.0 0.0 CDH24 5.9 7.2 8.3 9 0.0 0.0 0.0 0.0 Table S1: EC-BioID transmembrane protein hits. -

An Efficient Method for Native Protein Purification in the Selected Range from Prostate Cancer Tissue Digests
Original Article Page 1 of 7 An efficient method for native protein purification in the selected range from prostate cancer tissue digests Rumana Ahmad1,2, Carrie D. Nicora3, Anil K. Shukla3, Richard D. Smith3, Wei-Jun Qian3, Alvin Y. Liu1,2 1Department of Urology, University of Washington, Seattle, WA 98195, USA; 2Institute for Stem Cell and Regenerative Medicine, University of Washington, Seattle, WA 98195, USA; 3Biological Science Division and Environmental Molecular Sciences Laboratory, Pacific Northwest National Laboratory, Richland, WA 99352, USA Contributions: (I) Conception and design: AY Liu; (II) Administrative support: AY Liu; (III) Provision of study materials or patients: R Ahmad; (IV) Collection and assembly of data: R Ahmad; (V) Data analysis and interpretation: R Ahmad, CD Nicora, AK Shukla, RD Smith, WJ Qian; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors. Correspondence to: Rumana Ahmad. Department of Biochemistry, Era’s Lucknow Medical College & Hospital, Sarfarazganj, Hardoi Road, Lucknow-226003, UP, India. Email: [email protected]. Background: Prostate cancer (CP) cells differ from their normal counterpart in gene expression. Genes encoding secreted or extracellular proteins with increased expression in CP may serve as potential biomarkers. For their detection and quantification, assays based on monoclonal antibodies are best suited for development in a clinical setting. One approach to obtain antibodies is to use recombinant proteins as immunogen. However, the synthesis of recombinant protein for each identified candidate is time-consuming and expensive. It is also not practical to generate high quality antibodies to all identified candidates individually. Furthermore, non-native forms (e.g., recombinant) of proteins may not always lead to useful antibodies. -
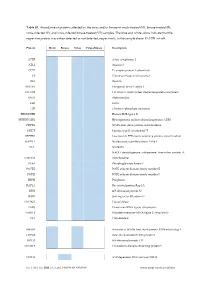
Virus-Infected (V), and Virus-Infected Binase-Treated (VB) Samples
Table S1. Abundance of proteins affected by the virus and/or binase in mock-treated (M), binase-treated (B), virus-infected (V), and virus-infected binase-treated (VB) samples. The blue and white colors indicate that the respective protein was either detected or not detected, respectively, in the sample above 1% FDR cut-off. Protein Mock Binase Virus Virus+Binase Description ACTB Actin, cytoplasmic 1 ATL2 Atlastin-2 CCT7 T-complex protein 1 subunit eta CS Citrate synthase, mitochondrial DES Desmin EEF1A1 Elongation factor 1-alpha 1 EFTUD2 116 kDa U5 small nuclear ribonucleoprotein component ENO1 Alpha-enolase EZR Ezrin GPI Glucose-6-phosphate isomerase HIST1H2BB Histone H2B type 1-B HNRNPA2B1 Heterogeneous nuclear ribonucleoproteins A2/B1 HSPD1 60 kDa heat shock protein, mitochondrial KRT75 Keratin, type II cytoskeletal 75 LRPPRC Leucine-rich PPR motif-containing protein, mitochondrial NAP1L1 Nucleosome assembly protein 1-like 1 NCL Nucleolin NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, NDUFS8 mitochondrial PGK1 Phosphoglycerate kinase 1 POTEE POTE ankyrin domain family member E POTEI POTE ankyrin domain family member I PRPH Peripherin RAP1A Ras-related protein Rap-1A RPS2 40S ribosomal protein S2 SF3B1 Splicing factor 3B subunit 1 TALDO1 Transaldolase TARS Threonine--tRNA ligase, cytoplasmic TARSL2 Probable threonine--tRNA ligase 2, cytoplasmic TKT Transketolase AHSA1 Activator of 90 kDa heat shock protein ATPase homolog 1 HSPA2 Heat shock-related 70 kDa protein 2 RPL15 60S ribosomal protein L15 TXNDC5 Thioredoxin domain-containing protein 5 DDX18 ATP-dependent RNA helicase DDX18 Int. J. Mol. Sci. 2020, 21, x; doi: FOR PEER REVIEW www.mdpi.com/journal/ijms Int.