Study of Germination Techniques for Helleborus Niger
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

HELLEBORUS NIGER (Hell. Nig.) Botanical Name : Helleborus Niger
HELLEBORUS NIGER (Hell. nig.) Botanical name : Helleborus niger Linn. Family: Ranunculaceae Synonyms : Elleborum nigrum, Helleborus grandiflorus Salisb., Veratrum nigrum Salisb. Common names : Hindi: Khorasani kutki; English: Black hellebore, Christmas rose; French: Ellebore noir; German: Sohwarze Uieswurzel. Description : A perennial, having brownish-black, knotted, brittle, fleshy rhizome, 2.5 to 7.5 cm long, 6 to 12 mm thick. Leaves on long stalks, which spring directly from the root. Stalks are cylindrical, tapering, smooth, shinning and pale green, mottled with red. Leaves pedate, deeply divided into several nearly separate lobes, coarsely seriate in the upper part, dark green above, paler beneath. Flowers on a scape shorter than petiole, at first pinkish-white, becoming green. Macroscopical : The drug occurs in irregularly branched, blackish pieces from 3.0 to 6.0 cm in length and from 5 to 8 mm in diameter. The branches show encircling leaf scars and the remains of the aerial stem or buds. Microscopical : Transverse sections of the rhizome shows considerable variations, 4 to 12 or more vascular bundles often of widely different shapes. Habitat : Found in alpine regions. History and authority : Introduced by Hahnemann in 1805. Allen’s Encyclop. Mat. Med. Vol. IV, 547. Part used : Rhizome. Moisture content of fresh rhizome 200 ml per 100 g solids. Preparation : (a) Mother Tincture φ Drug strength 1/10 Helleborus Niger in coarse powder 100 g Purified Water 400 ml Strong Alcohol 635 ml to make one thousand millilitres of the Mother Tincture. (b) Potencies: 2x contain one part tincture, three parts purified water and six parts Strong Alcohol. 3x and higher with Dispensing Alcohol. -

Helleborus Niger Helleborus Niger, Commonly Called Christmas Rose Or Black Hellebore, Is an Evergreen Perennial Flowering Plant in the Buttercup Family, Ranunculaceae
Helleborus niger Helleborus niger, commonly called Christmas rose or black hellebore, is an evergreen perennial flowering plant in the buttercup family, Ranunculaceae. It is poisonous. Although the flowers resemble wild roses (and despite its common name), Christmas rose does not belong to the rose family (Rosaceae). Taxonomy The black hellebore was described by Carl Linnaeus in volume one of his Species Plantarum in 1753.The Latin specific name niger (black) may refer to the colour of the roots. There are two subspecies: H. niger niger and H. niger macranthus, which has larger flowers (up to 3.75 in/9 cm across). In the wild, H. niger niger is generally found in mountainous areas in Switzerland, southern Germany, Austria, Slovenia, Croatia and northern Italy. Helleborus niger macranthus is found only in northern Italy and possibly adjoining parts of Slovenia. Description Helleborus niger is an evergreen plant with dark leathery pedate leaves carried on stems 9–12 in (23–30 cm) tall. The large flat flowers, borne on short stems from midwinter to early spring, are generally white, but occasionally purple or pink. The tips of the petals may be flushed pink or green, and there is a prominent central boss of yellow. Horticulture The plant is a traditional cottage garden favourite because it flowers in the depths of winter. Large- flowered cultivars are available, as are pink-flowered and double-floweredselections. It can be difficult to grow well; acid soil is unsuitable, as are poor, dry conditions and full sun. Moist, humus-rich, alkaline soil in dappled shade is preferable. Leaf-mould can be dug in to improve heavy clay or light sandy soils; lime can be added to 'sweeten' acid soils. -

In Vitro Propagation of Rosa 'Konstancin'
FOLIA HORTICULTURAE Folia Hort. 30(2), 2018, 259-267 Published by the Polish Society DOI: 10.2478/fhort-2018-0022 for Horticultural Science since 1989 ORIGINAL ARTICLE Open access www.foliahort.ogr.ur.krakow.pl In vitro propagation of Rosa ‘Konstancin’ (R. rugosa × R. beggeriana), a plant with high nutritional and pro-health value Agnieszka Wojtania*, Bożena Matysiak Department of Applied Biology Research Institute of Horticulture Konstytucji 3 Maja 1/3, 96-100 Skierniewice, Poland ABSTRACT The aim of the study was to develop an efficient micropropagation system forRosa ‘Konstancin’, an interspecific hybrid between R. rugosa and R. beggeriana, whose fruits have high pro-health value. Shoot cultures were initiated from shoot buds collected in May and August from 15-year-old field-grownRosa ‘Konstancin’ shrubs. The effect and interaction of different concentrations of phytohormones, sucrose and iron sources on in vitro initiation, multiplication and rooting of shoots were studied. The time of collecting explants from donor plants significantly affected the initiation of shoot culture ofRosa ‘Konstancin’. Considerably higher frequency of bud break (100%) was obtained in explants isolated in August as compared to those collected at the end of May (30%). All buds developed into single shoots after 2-4 weeks of growing on the basal Murashige and Skoog medium containing 2.2 µM BAP, 0.3 µM GA3 and 88 mM of sucrose. The highest multiplication rate (4.8 shoots/explant) in a 5-week period was obtained on MS medium containing 50% of nitrogen salts, 3.1 µM BAP, 0.9 µM GA3 and 58 mM sucrose. -
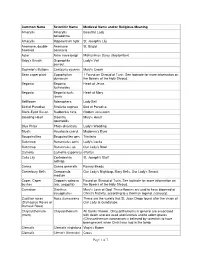
Of 7 Common Name Scientific Name Medieval Name And/Or Religious Meaning Amaryllis Amaryllis Belladonna Beautiful Lady
Common Name Scientific Name Medieval Name and/or Religious Meaning Amaryllis Amaryllis Beautiful Lady belladonna Amaryllis Hippeastrum hybr. St. Joseph's Lily Anemone, double- Anemone St. Brigid flowered coronaria Aster Aster nova-belgii Michaelmas Daisy (September) Baby's Breath Gypsophila Lady's Veil panicul. Bachelor's Buttons Centauria cyannis Mary's Crown Bean caper plant Zygophyllum ? Found on Shroud of Turin. See footnote for more information on dumosum the flowers of the Holy Shroud. Begonia Begonia Heart of Jesus fuchsioides Begonia Begonia fuch. Heart of Mary rosea Bellflower Adenophera Lady Bell Bird of Paradise Streliztia reginae Bird of Paradise Black-Eyed Susan Rudbeckia hirta Golden Jerusalem Bleeding Heart Dicentra Mary's Heart spectabilis Blue Phlox Phlox divaricata Lady's Wedding Bluets Houstonia caerul. Madonna's Eyes Bougainvillea Bougainvillea gen. Trinitaria Buttercup Ranunculus acris Lady's Locks Buttercup Ranunculus sp. Our Lady's Bowl Camelia Camellia (japonica) (Purity) Calla Lily Zantedeshia St. Joseph's Staff aethiop. Canna Canna generalis Rosary Beads Canterbury Bells Campanula Our Lady's Nightcap, Mary Bells, Our Lady's Smock medium Caper, Caper Capparis spinosa Found on Shroud of Turin. See footnote for more information on bushes (var. aegyptia) the flowers of the Holy Shroud. Carnation Dianthus Mary's Love of God. These flowers are said to have bloomed at caryophyllus Christ's Nativity, according to a German legend. (January) Castilian roses Rosa damascena These are the variety that St. Juan Diego found after the vision of (Damascus Roses or Our Lady at Guadalupe. Damask Rose) Chrysanthemum Chrysanthemum All Saints' Flower. Chrysanthemums in general are associated (mum) with death and are used and funerals and to adorn graves (Chrysanthemum coronarium is believed by scientists to have been present when Christ was laid in the tomb. -

Master Plant List
MASTER PLANT LIST 5 N 9 7 8 6 Glasshouse 4 Green Roof 1 2 3 7 MASTER PLANT LIST PAGE 1 TREES 4 Acer griseum PAPERBARK MAPLE 2 3 Acer palmatum ‘Atropurpureum Dissectum’ RED WEEPING CUT-LEAF JAPANESE MAPLE 3 4 5 6 7 Acer palmatum ‘Sango Kaku’ CORAL BARK JAPANESE MAPLE 7 Chamaecyparis nootkatensis ‘Pendula’ WEEPING NOOTKA CYPRESS 7 Chamaecyparis obtusa ‘Gracilis’ SLENDER HINOKI CYPRESS 1 6 Cornus rutgersensis ‘Celestial’ CELESTIAL DOGWOOD 3 6 Davidia involucrata ‘Sonoma’ SONOMA DOVE TREE 4 Gleditsia triacanthos inermis ‘Shademaster’ SHADEMASTER HONEY LOCUST 7 Magnolia grandiflora ‘Teddy Bear’ TEDDY BEAR MAGNOLIA 7 Magnolia grandiflora ‘Bracken’s Brown Beauty’ BRAKEN’S BROWN BEAUTY MAGNOLIA 3 Picea pungens ‘Iseli Fastigiate’ ISELI FASTIGIATE SPRUCE 3 7 Sciadopitys verticillata ‘Wintergreen’ WINTERGREEN UMBRELLA PINE 2 3 Stewartia pseudocamellia JAPANESE STEWARTIA 7 Thuja plicata ‘Atrovirens’ WESTERN RED CEDAR SHRUBS 8 Arbutus compacta DWARF STRAWBERRY TREE 7 Aucuba japonica ‘Rozannie’ ROSANNIE AUCUBA 7 Berberis x gladwynensis ‘William Penn’ BARBERRY 5 Buxus microphylla ‘Wintergreen’ BOXWOOD 8 Callicarpa ‘Profusion’ BEAUTY BERRY 5 7 Camellia sasanqua ‘Yuletide’ YULETIDE CAMELLIA 3 Camellia sasanqua ‘Setsugekka’ SETSUGEKKA CAMELLIA 5 Chaenomeles ‘Dragon’s Blood’ QUINCE 5 Chaenomeles ‘Scarlet Storm’ QUINCE 5 Cornus sericea ‘Bud’s Yellow’ YELLOWTWIG DOGWOOD 1 Corylus avellana ‘Contorta’ HARRY LAUDER’S WALKING STICK 6 Cryptomeria japonica ‘Black Dragon’ BLACK DRAGON JAPANESE CEDAR 8 Cotoneaster dammeri BEARBERRY 2 Daphne genkwa LILAC DAPHNE 4 Dichroa febrifuga CHINESE QUININE 2 Edgeworthia chrysantha ‘Snow Cream’ RICE PAPER SHRUB 7 Fatshedera lizei TREE IVY 7 x Fatshedera lizei ‘Variegata’ VARIGATED TREE IVY 5 Fothergilla gardenii DWARF WITCH ALDER 5 Hamamelis japonica ‘Shibamichi Red’ JAPANESE WITCH HAZEL 2 4 Hydrangea macrophylla ssp. -

Plants Toxic to Horses
Plants Toxic to Horses Adam-and-Eve (Arum, Lord-and-Ladies, Wake Robin, Starch Root, Bobbins, Cuckoo Plant) | Scientific Names: Arum maculatum | Family: Araceae African Wonder Tree () | Scientific Names: Ricinus communis | Family: Alocasia (Elephant's Ear) | Scientific Names: Alocasia spp. | Family: Araceae Aloe () | Scientific Names: Aloe vera | Family: Liliaceae Alsike Clover () | Scientific Names: Trifolium hybridum | Family: Leguminosae Amaryllis (Many, including: Belladonna lily, Saint Joseph lily, Cape Belladonna, Naked Lady) | Scientific Names: Amaryllis spp. | Family:Amaryllidaceae Ambrosia Mexicana (Jerusalem Oak, Feather Geranium) | Scientific Names: Chenopodium botrys | Family: Chenopodiaceae American Bittersweet (Bittersweet, Waxwork, Shrubby Bittersweet, False Bittersweet, Climbing Bittersweet) | Scientific Names: Celastrus scandens| Family: Celastraceae American Holly (English Holly, European Holly, Oregon Holly, Inkberry, Winterberry) | Scientific Names: Ilex opaca | Family: Aquifoliaceae American Mandrake (Mayapple, Indian Apple Root, Umbrella Leaf, Wild Lemon, Hog Apple, Duck's Foot, Raccoonberry) | Scientific Names:Podophyllum peltatum | Family: Berberidaceae American Yew (Canada Yew, Canadian Yew) | Scientific Names: Taxus canadensus | Family: Taxaceae Andromeda Japonica (Pieris, Lily-of-the-Valley Bush) | Scientific Names: Pieris japonica | Family: Ericaceae Angelica Tree (Hercules' Club, Devil's Walking Stick, Prickly Ash, Prickly Elder) | Scientific Names: Aralia spinosa | Family: Araliaceae Apple (Includes crabapples) -

POISONOUS PLANTS for PEOPLE Commonly Know As: Scientific Name: Poisonous Parts: Aconite;Monkshood;Wolfbane Aconitum All Parts Alstromeria Alstroemeria Spp
Fred Hall-CEA Agriculture/NR 600 Scott Street, Suite 200 Wichita Falls, Texas 76301 POISONOUS PLANTS FOR PEOPLE Commonly know as: Scientific Name: Poisonous Parts: Aconite;Monkshood;Wolfbane Aconitum All parts Alstromeria Alstroemeria spp. Leaves, stems (dermatitis) Angel Trumpet Brugmanisa All parts Apple Malus spp. Seeds in quantity Azalea; Rhododendron Rhododendron spp. All parts Belladonna Atropa belladonna All parts Bittersweet, Climbing or American Celastrus scandens Fruits Bittersweet; Nightshade Solanum dulcamara Stems, leave, berries Bleeding Heart Dicentra spp. All parts Bluebonnets Lupinus spp. All parts Bracken Fern Pteridium aquilinum All parts Calla Lily Calla spp. All parts Carnation Dianthus caryophyllus All parts Carolina Jessamine, Yellow Glesemium sepmervirens All parts Castor Bean Ricinus communis Foliage, seeds China Berry Melia azedarach Berries Caladium Caladium spp. Leaves, roots Corn poppy:Red poppy;Field poppy Papaver rhoeas All parts Crocus, Autumn; Meadow Saffron Colchicum autumnal All parts Cyclamen Cyclamen spp. Roots Delphinium; Larkspur Delphinium spp. All parts Dumb cane Dieffenbachia spp. All parts Elephant Ear Philodendron spp. All parts Flame Lily Gloriosa superba All parts Four-O Clock Mirabilis spp. Roots, seeds Foxglove; Digitalis Digitalis purpurea All parts Colden Chain Tree Laburnum anagyroides Fruits Hellebore, White Veratrum album All parts Hellebore; Christmas Rose Helleborus niger All parts Henbane, Black Hyoscyamus niger All parts Holly, English Hex aquifolium Berries Commonly know as: Scientific Name: Poisonous Parts: Hyacinth Hyacinths spp. All parts Iris Iris spp. Underground stems, leaves Ivy, English Hedera helix Leaves, berries Jerusalem Cherry Solanum pseudo capsicum Leaves, berries Jimsonweed, Thorn-Apple, Datura Datura stramonium & spp. All parts Lantana Lantana camara Young berries Laurel Cherry, Spp. -

Are Riding High
*16_emergcrops.qxd 5/1/06 1:24 PM Page 16 emergingcrops Hellebores Are Riding High Hellebores are long-lived, low-maintenance, and drought- and deer-resistant plants that flower in a wide range of colors from winter to early spring. By Alan Bush here would be little exaggeration if you said hellebores de- By consistently improving served a spot on the varieties, plant breeders current hit parade of perennials.T The rapid ascendancy have been able to refine to mainstream popularity has even and reinvent some species. begun to shake the pillars of the hosta world and its long-lasting These old standbys have claim to dominion over all in the shade garden. been transformed from Why are hellebores moving up troublemakers to industry so quickly in sun-challenged gar- dens? They flower in a wide range sweethearts, from plants of colors from winter to early no one wanted to grow spring and also tolerate heat. They are long lived, low maintenance, to ones they can’t keep and drought and deer resistant. Most commercially available plants in stock. To show the are cold hardy from at least Zone 4 potential of these through Zone 9. In their native provenance of Southern Europe, emerging crops, GPN is the species experiences warm days running a 6-part series and cool evenings, yet they have proven very adaptable, surviving detailing each crop’s even in the southern United States. transformation and some Sound like a recipe for success? tips for success. Breeding Better Plants The ordinary species — and even some of the earlier hybrids — January: Lobelia did little to excite home gardeners. -

HELLEBORUS (Hellebore, Lenten Rose)
HELLEBORUS (Hellebore, Lenten Rose) Rachel Hutchins and Melinda Zoehrer Beautiful and easy to grow, gardeners find winter-flowering hellebores or Lenten Rose are an enchanting plant for the shade garden and a harbinger of spring. Primarily native to Europe, the evergreen perennial comes in a variety of exquisite saucer-shaped, 2–4 inch flowers from flushed pinks to buttery Helleborus ‘Black Tie Affair’ Helleborus ‘First Dance’ hues to deep velvety black shades that can last 6–8 weeks. Hellebores’ serrated foliage rises on sturdy stems and often remains attractive throughout the year. Historically used as both a poison and a purgative, hellebores are deer and rabbit resistant due to their toxicity. Although known for their drought tolerance once established and the ability to survive neglect, hellebores grow best in evenly moist, well-drained soil. They are very sensitive to poor drainage. They can withstand a few hours of morning or late afternoon sun but perform best in part-shade or part-sun. Hellebores are a delightful addition to any partially shaded border or patio where their flowers Helleborus ‘BLT 02’ and foliage are on display when much of the landscape is still Helleborus ‘Flower Girl’ shades of brown and gray. The list below contains many new and beloved hellebore cultivars to add to any collection. Latin Name Common Name Mature Size Light Soil Pot Size Price Helleborus ‘Black Tie Affair’ 1.5 88 d qt $12 Dark violet edge, violet veins overlaid on cream-colored double flowers; yellow stamens; early–midspring. Helleborus ‘BLT02’ Angel Glow Christmas Rose 1.5 88 d 1 g $16 Pink buds open frosty, light pink flowers, green tones in age; midwinter–early spring; Helleborus ‘Blushing Bridesmaid’ foliage frosty blue-green; compact, mounded habit. -

Helleborus Niger Helleborus Niger, Commonly Called Christmas Rose Or Black Hellebore, Is an Evergreen Perennial Flowering Plant in the Buttercup Family, Ranunculaceae
Helleborus niger Helleborus niger, commonly called Christmas rose or black hellebore, is an evergreen perennial flowering plant in the buttercup family, Ranunculaceae. It is poisonous. Although the flowers resemble wild roses (and despite its common name), Christmas rose does not belong to the rose family (Rosaceae). Taxonomy The black hellebore was described by Carl Linnaeus in volume one of his Species Plantarum in 1753.The Latin specific name niger (black) may refer to the colour of the roots. There are two subspecies: H. niger niger and H. niger macranthus, which has larger flowers (up to 3.75 in/9 cm across). In the wild, H. niger niger is generally found in mountainous areas in Switzerland, southern Germany, Austria, Slovenia, Croatia and northern Italy. Helleborus niger macranthus is found only in northern Italy and possibly adjoining parts of Slovenia. Description Helleborus niger is an evergreen plant with dark leathery pedate leaves carried on stems 9–12 in (23–30 cm) tall. The large flat flowers, borne on short stems from midwinter to early spring, are generally white, but occasionally purple or pink. The tips of the petals may be flushed pink or green, and there is a prominent central boss of yellow. Horticulture The plant is a traditional cottage garden favourite because it flowers in the depths of winter. Large- flowered cultivars are available, as are pink-flowered and double-floweredselections. It can be difficult to grow well; acid soil is unsuitable, as are poor, dry conditions and full sun. Moist, humus-rich, alkaline soil in dappled shade is preferable. Leaf-mould can be dug in to improve heavy clay or light sandy soils; lime can be added to 'sweeten' acid soils. -

Medieval Plant Names and Modern Corollaries
Medieval plant names and modern corollaries This is the general listing from the Cloisters Gardens, Fort Tyron Park, New York, New York, 10040." For recent diagrams of the gardens and lists of the plants grown in each year please write to them directly. Latin Name Modern Name Medieval Name Achillea millefolium Yarrow Our Lord's Back Aconitum napellus Monkshood Mary's Slipper Adonis aestivalis Pheasant's Eye Mary's Rose Agrimonia eupatoria Agrimony Our Lord's Nail Agrostemma guthago Corn Cockle Mary's Rose Aguga reptans Bugle St. Lawrence Plant Alcea rosea Hollyhock St. Josephs Staff Alchemilla vulgaris Lady's Mantle Mary's Mantle Allium schoenoprasum Chives Our Lady's Garleek Althea officinalis Marshmallow Our Lady's Cheeses Anagallis arvensis Scarlet Pimpernel Ladybird Anemone coronaria Poppy Anemone Flower of Field Anemone pulsatilla Pasque Flower Pasque Flower Anethum graveolens Dill Devil-Away Angelica archancelica Angelica Angel's Plant Anthemis tinctoria Golden Marguerite St. John's Flower Antirrhinum majus Snapdragon Infant Jesus Shoes Apium graveolens Wild Celery Virgin's Marsh Platter Aquilegia vulgaris Columbine Our Lady's Shoes Armeria maritime Thrift, Sea Pink Our Ladies Pincushion Artemisia abrotanum Southernwood Our Lord's Wood Artemisia absinthium Wormwood Mary's Tree Artemisia camphorata Camphor Artemisia Our Lady's Maid Arum maculatum Cuckoo-Pint Lady-Lords Atropa belladonna Deadly Nightshade Beautiful Lady Bellis perennis English Daisy Mary's Rose Borago officinalis Borage Virgin's Face Bryonica dioica Red Bryony Our Lady's Signet Brassica nigra Black Mustard Mustard Seed Calendula officinalis Pot Marigold Marygold Campanula medium Canterbury Bells Our Ladies Nightcap Carlina acaulis Charlemagne’s Mary's Thistle Centauria cyanis Cornflower Mary's Crown Cheiranthus cheiri Wall Flower Mary's Flower Chelidonium majus Celandine Gift of God Chenopodium botrys Jerusalem Oak Jerusalem Cabbage Chrysanthemun bals.tan. -

History and Update on the World of Hellebores©
History and Update on the World of Hellebores 585 History and Update on the World of Hellebores© John E. Elsley Song Sparrow, 5200 Bryte St., Greenwood, South Carolina 29649 Email: [email protected] HELLEBORES: SPECIES AND IMPORTANT EARLY HYBRIDIZERS A working knowledge of the available species is of major importance in breeding programs. Sowing species seed provides potential hybridizers with variable prog- eny from which can be selected potential parental individuals displaying desirable characteristics. In the case of hellebores, these characteristics include both floral and foliar features combined with overall vigor. SPECIES Helleborus argutifolius. Native southern Europe/Corsica. Handsome caules- cent specimen. A superb performer in the Pacific Northwest. Magnificent, variable foliage. The bold foliage creates an attractive contrast with the heavy effect of conifer foliage, i.e., Thuja. Cultivars: ‘Janet Starnes’, speckled cream leaves; ‘Pacific Frost’, heavy cream foli- age speckling. Helleborus foetidus. Native to central Europe. Caulescent species with handsome variable foliage and terminal clusters of pendulous bell-shaped flowers. Short lived but self-seeds freely. Often flowers in second year from seed—blooms late winter, early spring. Cultivars: ‘Sienna’, dark green foliage and chartreuse flowers. Selected by Will McLewin—noted English species authority; Wester Flisk Group, red-stemmed form from east Scotland (Seeding progeny quickly looses this desirable trait if grown with non-red-stemmed forms. Doubtful if the original form exists; most are now selections from seedling strains.); Red Silver strain, a Wester Flisk Group cross with silvered leaves and reddish stems, Northwest Garden Nursery introduction; ‘Gold Bullion’ a variable seed strain with yellow foliage. Helleborus multifidus.