Oom O#00Noo-O
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

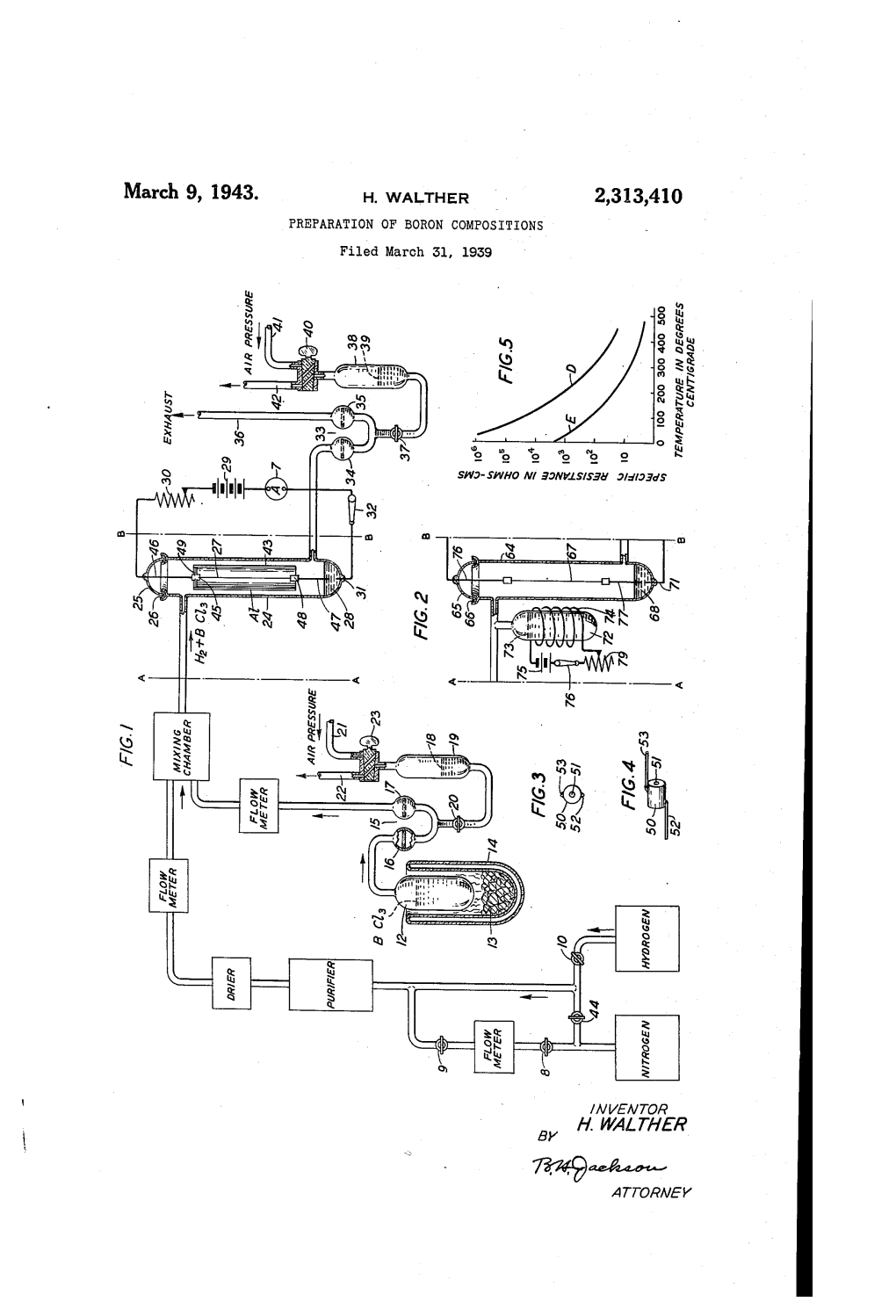
United States Patent Office Patented May 9, 1961 1
2,983,583 United States Patent Office Patented May 9, 1961 1. 2 of the tube and drained into the hot Zone. Most of the 2,983,583 silicon tetrachloride was recovered unchanged. 0.43 millimole of BCls were obtained. Based on the SiCl4 METHOD OF PREPARNG BORON TRICHLORDE consumed, the yield of boron trichloride was about 70% FROM BORIC OXDE AND SILICON TETRA 5 based on Equation 2. CHLORDE Example III-In another experiment, conducted in William H. Schechter, Bradford Woods, Pa., assignor to a manner similar to those above, 10.0 millimoles of S2Cl2 Callery Chemical Company, Pittsburgh, Pa., a corpor was heated with 5.04 millimoles of BOs at 800° C. for ration of Pennsylvania 10 minutes. Boron trichloride and sulfur dioxide were O obtained in the volatile products, and a yellow solid, be No Drawing. Filed Mar. 28, 1958, Ser. No. 725,471 lieved to be sulfur, formed in the tube. 2 Claims. (CI. 23-205) Example IV.-9.32 millimoles of PC were passed over excess BO heated to 800° C. for 10 minutes. The vola This invention relates to the preparation of boron tri 15 tile products formed were analyzed with an infrared spec chloride and more particularly to the preparation of trometer and found to be predominantly BCl3. Some boron trichloride from boric oxide and non-metallic orange colored solids also formed in the reactor during chlorides. the reaction. The non-metallic chlorides which have been found Boron trichloride, BC, is used in several processes to useful in the practice of this invention are all volatile prepare other boron compounds, as a catalyst, and, in 20 liquids, at ordinary temperatures, and their reaction with general, is regarded as a basic boron compound. -

Boron and Titanium(IV) Halide Mediated Reactions
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 8-2010 Boron and Titanium(IV) Halide Mediated Reactions Michael Patrick Quinn University of Tennessee - Knoxville, [email protected] Follow this and additional works at: https://trace.tennessee.edu/utk_graddiss Part of the Organic Chemistry Commons Recommended Citation Quinn, Michael Patrick, "Boron and Titanium(IV) Halide Mediated Reactions. " PhD diss., University of Tennessee, 2010. https://trace.tennessee.edu/utk_graddiss/908 This Dissertation is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Michael Patrick Quinn entitled "Boron and Titanium(IV) Halide Mediated Reactions." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Chemistry. George W. Kabalka, Major Professor We have read this dissertation and recommend its acceptance: Shane Foister, Ziling (Ben) Xue, Paul Dalhaimer Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a dissertation written by Michael Patrick Quinn entitled ―Boron and Titanium(IV) Halide Mediated Reactions.‖ I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Chemistry. -

Boron Trichloride. Bcl₃
→ Datasheet Boron trichloride. BCl₃ Product information BCl₃ is the main gas composition in High-K and metal anisotropic plasma etch. Atomic layer etch and pulsed plasma etch. BCl₃ is mainly used for fine etching of aluminum circuits in the manuf- acturing process of LCD panels. Characteristics Liquefied gas, decomposes in water to hydrogen chloride and boric acid. Forms white fumes in humid air. Pungent odor. Highly corrosive. Gas density is heavier than air. Physical data Molecular weight [g/mol] 117.17 Boiling point at 1.013 bar [°C] 12.5 at 14.5 psi [°F] 54.52 at 1.013 bar, Density 5.162 at 1 atm., 70 °F [lb/ft³] 0.315 15 °C [kg/m³] Vapor pressure at 0 °C [bar] 0.63 at 32 °F [psi] 9.09 at 20 °C [bar] 1.33 at 70 °F [psi] 19.91 Flammability range in air (% volume) Non-combustible Product specification Purity grade Typical purity Typical impurities [ppm] N₂ O₂+Ar CO CO₂ COCl₂ CH₄ HCl 5.0N ≥99.999 % ≤4 ≤1 ≤0.5 ≤1 ≤0.5 ≤0.5 ≤50 5.5N ≥99.99995 % ≤1 ≤0.5 ≤0.5 ≤1 ≤0.5 ≤0.5 ≤25 Contact our team for higher grade or different specification products. Shipping information UN number CAS number EC number DOT label Hazard labels required 1741 10294-34-5 233-658-4 Poison gas ADR Class 2, 2 TC DOT Class 2.3 → Boron trichloride. Product datasheet. Page 2 Packaging information Package Cylinder Cylinder Cylinder Cylinder Cylinder Cylinder Fill Pressure Valve Valve designa- internal material diameter height to tare weight contents (psig) outlet material options o tion volume valve outlet @ 70 F US Cylinder 209 44 L Nickel 9 in 52 in 130 lb 110 lb 4.4 CGA -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Heat of Formation of Boron Trichloride Walter H
Journal of Research of the National Bureau of Sta nda rds Vol. 62, No.5, May 1959 Research Paper 2956 Heat of Formation of Boron Trichloride Walter H. Johnson, Richard G . Miller, ! a nd Edward J. Prosen The heat of formation of gaseou boron t richloride has been determined by t he direct reaction of gaseous chlorine with amorphous boron in a calorimeter. B (amorph) + 3/2 CIz (gas) = BCh(gas) AHfO(25° C)= - 407.98 ± 1.34 kj/mole (- 97.51 ± 0.32 kcal/moJe). By utilizing t he valu es previously reported for the hea ts of formation of boric acid, dibor ane, and pentaborane, t he heat of hydrolysis of boron t richloride and the heats of reac tion of dibora ne a nd pentaboran e wit h chlorine have been obtained. By the use of an estimat ed value for t he heat of sublimation of boron, the average bond energy of t he B- CI bond in boron t richloride is found to be 105. 2 k cal at 0° K . The data on the heats of formation of diborane, boric oxide, boric acid, and boron trichloride now form a co n sistent set of values. I. Introduction 3. Materials and Apparatus The heat of formation of boron trichloride has The boron was prepared by the thermal de compo long been uncertain because of the fact that calcu sition of diborane by pa sing diborane diluted with lation of it has been dependent upon uncertain values helium through a quartz tube heated to 600 0 C [1] . -

R Ysssssssss
Nov. 5, 1957 w A. R. GLOBUS 2,812,240 PROCESS OF MAKING BORON NITRIDE Filed Feb. 17, 1955 sS s : r N 1 - a 4th 8 R ... SNs S. s INVENTOR SSat man avia as a awarar ar.Kettes. N N: aAAAACsy A, GZOAAS NA s : RN A... 6 YSSSSSSSSSa gram ATTORNEY 2,812,240 United States Patent Office Patented Nov. 5, 1957 2 through valvcd pipe 39 to pump 41, by which the mixture 2,812,240 is forced through pipe 42 and vertical section 43 back inio the lower end of reactor . Heat is supplied by PROCESS OF MAKING BORON NITRIDE In eans of the elix 45 to keep the vertical section 43 at Alfed R. Globus, Forest Hills, N. Y., assignor to United a temperature above the melting point of the metal. ternational Research, Inc., a corporation of New The nitrogen introduced with the boron trichloride should be at least Sioichiometrically sufficient to react York with the horon prescrlt in the trichloride, with formation Application February 17, 1955, Serial No. 488,920 of boron nitride (BN). In practice a substantial excess 1 Claim. (C. 23-191) O of nitrogen will he used to insure completion of the de sired reaction and the maintenance cf a nitrogen atmos This invention is a new and useful process of making phere in the free space in the upper cnd of reactor 1. boron nitride and will be fully understood from the fol The boron trichloride will react immediately with the lowing description read in conjunction with the drawing allininum, with production of aluminum chloride (AlCl3) in which: and boron, which boron reacts immediately with the Fig. -

THE MONATOMIC IONS! 1. What Is the Formula for Silver? Ag 2. What Is
Name: ______________________________ THE MONATOMIC IONS! 1. What is the formula for silver? Ag+ 22. What is the formula for cobalt (II)? Co2+ 2. What is the formula for cadmium? Cd2+ 23. What is the formula for chromium (II)? Cr2+ 3. What is the formula for manganese (II)? Mn2+ 24. What is the formula for copper (II)? Cu2+ 4. What is the formula for nickel (II)? Ni2+ 25. What is the formula for tin (IV)? Sn4+ 5. What is the formula for chromous? Cr2+ 26. What is the formula for lead (IV)? Pb4+ 6. What is the formula for zinc? Zn2+ 27. What is the formula for iron (III)? Fe3+ 2+ 2+ 7. What is the formula for cobaltous? Co 28. What is the formula for mercury (I)? Hg2 8. What is the formula for cuprous? Cu+ 29. What is the formula for lead (II)? Pb2+ 9. What is the formula for ferrous? Fe2+ 30. What is the formula for mercury (II)? Hg2+ 2+ 2+ 10. What is the formula for mercurous? Hg2 31. What is the formula for iron (II)? Fe 11. What is the formula for stannous? Sn2+ 32. What is the formula for copper (I)? Cu+ 12. What is the formula for plumbous? Pb2+ 33. What is the formula for tin (II)? Sn2+ 13. What is the formula for chromic? Cr3+ 34. What is the formula for fluoride? F- 14. What is the formula for cobaltic? Co3+ 35. What is the formula for chloride? Cl- 15. What is the formula for cupric? Cu2+ 36. What is the formula for hydride? H- 16. -

A New Multifunctional Initiator System for the Living Cationic Polymerization of Vinyl Ethers
472 Macromol. Rapid Commun. 21, 472–475 (2000) Communication: Combination of hexa(chloromethyl)- melamine (HCMM) and zinc chloride was found to be a multifunctional initiator system for the living cationic polymerization of isobutyl vinyl ether. HCMM was synthe- sized by reaction of hexa(methoxymethyl)melamine and boron trichloride. Characterization of the polymers by means of GPC and 1H NMR showed that initiation was rapid and quantitative and that the initiator is hexafunc- tional, leading to six-armed star-shaped polymers. A new multifunctional initiator system for the living cationic polymerization of vinyl ethers Xiaochun Zhang1, Eric J. Goethals*1, Ton Loontjens2, Frank Derks2 1 University of Gent, Department of Organic Chemistry, Polymer Chemistry Division, Krijgslaan 281(S4-bis), 9000 Gent, Belgium 2 DSM Research, P.O. Box, 6160 MD Geleen, The Netherlands (Received: January 19, 2000) Introduction diethyl ether (1.0 M) and BCl3 in hexane (1.0 M) were pur- chased from Aldrich and used without further treatment. For the synthesis of star-shaped polymers, two methods Hexa(methoxymethyl)melamine (HMMM) (Cymel 303) was 1–3) based on living polymerization have been described : purchased from American Cynamide and used without end-capping of linear living polymers with a multifunc- further purification. tional end-capper (“arm-first” method), and initiation of the polymerization with a multifunctional initiator system (“core-first” method). In case of living cationic polymeri- Synthesis of hexa(chloromethyl)melamine (HCMM) zation of vinyl ethers, star-shaped polymers have been A 250 ml double-cocked flask provided with rubber septum prepared by both methods4, 5). The core-first method has and magnetic stirring bar containing 4.0 g of HMMM was the advantage that, at the end of the polymerization, end- dried in vacuum at 408C for 1 h and then filled with dry capping of the living chain ends is still possible, thus pro- argon. -

Boron Trichloride
Material Safety Data Sheet 1. Product and Company Identification Product name : Boron Trichloride Chemical formula : BCl3 Synonyms : Trichloroborane Company : Specialty Gases of America, Inc 6055 Brent Dr. Toledo, OH 43611 Telephone : 419-729-7732 Emergency : 800-424-9300 2. Composition/Information on Ingredients Components CAS Number % Volume Boron Trichloride 10294-34-5 100% 3. Hazards Identification Emergency Overview Colorless poison gas with acidic odor. Nonflammable. Irritating and corrosive to the eyes, skin and respiratory system. Exposure to high concentrations may result in burns to mucous membranes. Inhalation into the deep lung may result in dangerous retention of body fluid and swelling in the lungs (pulmonary edema) and chemical pneumonitis. Hydrolyzes into hydrochloric and boric acid in the presence of water or moisture. Contents under pressure. Use and store below 125 F. Potential Health Effects Inhalation : Boron trichloride hydrolyzes very rapidly into hydrochloric acid and boric acid in the presence of moisture. Slight exposure results in irritation of the upper respiratory tract and cough. Higher concentrations may cause inflammation and congestion of the lungs. Inhalation into the deep lung may result in difficulty breathing, chest pain, chemical pneumonitis and fluid retention with swelling in the lungs (edema). May cause shock, coma and death. Eye contact : Eye contact will cause severe irritation, inflammation, and painful burns. Burns may result in lesions and blindness. PERSONS WITH POTENTIAL EXPOSURE TO BORON TRICHLORIDE SHOULD NOT WEAR CONTACT LENSES. Skin contact : Low concentrations may cause “stinging” of the skin. Severe burns may result at higher concentrations. Inorganic acid-like burns and corrosive action will occur at high concentrations resulting in lesions and early necrosis. -

Preparation of Diborane from Lithium Hydride and Boron Trihalide Ether Complexes1
OCt. 20, 1952 &BORANE FROM LITHIUMHYDRIDE AND BORONTRIHALIDE ETHER COMPLEXES 5047 [CONTRIBUTION FROM THE RESEARCH LABORATORYOF THE GENERALELECTRIC COMPANY] Preparation of Diborane from Lithium Hydride and Boron Trihalide Ether Complexes1 BY J. R. ELLIOTT,E. M. BOLDEBUCKAND G. F. ROEDEL RECEIVEDFEBRUARY 14, 1952 The preparation of diborane from lithium hydride and boron trihalide etherates under different conditions is described and secondary reactions are discussed. The reaction between lithium hydride and boron trifluoride in ethyl ether has been shown to proceed by two different courses. If ether-soluble, active hydrogen-containing promoters are present, or if pressure is used to force the reaction between lithium hydride and diborane, the hydride is converted completely to lithium borohydride and lithium fluoride before diborane is evolved. In the absence of soluble promoters, diborane escapes continually from the solution, lithium borofluoride is formed and lithium borohydride does not accumulate. If less than a specified ratio of boro- hydride to hydride exists in the ether solution, the borohydride is consumed in the continuing reaction, and diborane is evolved. In tetrahydrofuran, a promoter is not required for the conversion of lithium hydride to lithium borohydride. Since the solubility of diborane is relatively high in tetrahydrofuran, conditions favor the production of borohydride by reaction of diborane with lithium hydride, as in the pressure reaction with ether as solvent. Introduction continuously evolved at a mole rate -

Boron Trichloride Safety Data Sheet
Boron trichloride Safety Data Sheet P-4566 This SDS conforms to U.S. Code of Federal Regulations 29 CFR 1910.1200, Hazard Communication. Issue date: 01/01/1979 Revision date: 01/21/2021 Supersedes: 10/13/2016 Version: 1.0 SECTION: 1. Product and company identification 1.1. Product identifier Product form : Substance Substance name : Boron trichloride CAS-No. : 10294-34-5 Formula : BCl3 1.2. Relevant identified uses of the substance or mixture and uses advised against Use of the substance/mixture : Industrial use; Use as directed. 1.3. Details of the supplier of the safety data sheet Linde Inc. 10 Riverview Drive Danbury, CT 06810-6268 - USA www.lindeus.com Linde Inc. 1-844-44LINDE (1-844-445-4633) Linde Electronics 1-800-932-0624 or 1-908-329-9700 1.4. Emergency telephone number Emergency number : Onsite Emergency: 1-800-645-4633 CHEMTREC, 24hr/day 7days/week — Within USA: 1-800-424-9300, Outside USA: 001-703-527-3887 (collect calls accepted, Contract 17729) SECTION 2: Hazard identification 2.1. Classification of the substance or mixture GHS US classification Press. Gas (Liq.) H280 Acute Tox. 3 (Inhalation:gas) H331 Skin Corr. 1B H314 Eye Dam. 1 H318 STOT SE 3 H335 2.2. Label elements GHS US labeling Hazard pictograms (GHS US) : GHS04 GHS05 GHS06 Signal word (GHS US) : Danger Hazard statements (GHS US) : H280 - CONTAINS GAS UNDER PRESSURE; MAY EXPLODE IF HEATED H314 - CAUSES SEVERE SKIN BURNS AND EYE DAMAGE H331 - TOXIC IF INHALED CGA-HG22 - CORROSIVE TO THE RESPIRATORY TRACT CGA-HG01 - MAY CAUSE FROSTBITE. -

Modeling of the Isobutylene Polymerization Process in Agitated Reactors
MODELING OF THE ISOBUTYLENE POLYMERIZATION PROCESS IN AGITATED REACTORS by Yongtai Li Bachelor of Automation, Beijing University of Chemical Technology, 2007 Master of Process Control, Beijing University of Chemical Technology, 2010 Submitted to the Graduate Faculty of Swanson School of Engineering in partial fulfillment of the requirements for the degree of Master of Science in Petroleum Engineering University of Pittsburgh i 2016 2016 UNIVERSITY OF PITTSBURGH SWANSON SCHOOL OF ENGINEERING This thesis was presented by Yongtai Li It was defended on July 26, 2016 and approved by George E. Klinzing, PhD, Professor, Department of Chemical and Petroleum Engineering Robert M. Enick, PhD, Professor, Department of Chemical and Petroleum Engineering Thesis Advisor: Badie Morsi, PhD, Professor, Department of Chemical and Petroleum Engineering ii Copyright © by Yongtai Li 2016 iii MODELING OF THE ISOBUTYLENE POLYMERIZATION PROCESS IN AGITATED REACTORS Yongtai Li, M.S. University of Pittsburgh, 2016 In this study, a comprehensive model for the IBP process in agitated reactors was developed based on the reaction mechanism by Vasilenko et al. 2010, and takes into account the reaction rate kinetics for initiation, propagation, chain transfer, and chain termination steps as well as the mixing effects. The model coupled the mass balance equations for each reaction step with those of the segregated zones model for micro- and macro-mixing effects by Villermaux 1989, and was numerically solved by Matlab. The model was then used to predict the effect of various operating variables on the IBP process performance, in terms of the three main metrics: monomer conversion (X), number average molecular weight (Mn) and polydispersity index (PDI).