Assessment of Water Quality of Baitarani River
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Mahanadi Delta
Study Area - Mahanadi Delta Mahanadi delta is formed by a network of three major rivers: Mahanadi, Brahmhani and Baitarini draining into the Bay of Bengal. The coastline of the delta is about 200 km long which stretches from south near Chilika to north up to Dhamra River. Using SRTM 30m digital surface elevation data, area within 5m contour from the coast line has been extracted within the vicinity of Mahanadi delta (Figure 1). Figure 1 SRTM 5m filled contour (shown as light blue) overlaid on LULC map of Mahandi Delta The 5m contour spread across districts of Khordha, Puri, Jagatsinghpur, Kendrapara, Jajpur and Bhadrak. Chilika lake is also within 5m contour line and within the Mahanadi delta. The district Baleswar, in the extreme north is within 5m contour, but it is outside the Mahanadi delta. The geomorphological map (Figure 2) of the region (Source: Bhuvan-NRSC) also indicates that these features lie in coastal region of Mahanadi and adjacent river systems. Using the above criteria, five districts, namely, Khordha, Puri, Jagatsinghpur, Kendrapara and Bhadrak have been considered as the study area for Mahanadi Delta (Figure 3). The north and south bounds of the study areas have been defined using the geomorphological map, and Jajpur has been excluded, as it is not lying in the Mahanadi river system, though part of coastal zone. The total study area is 13137.679sq km and consisting of 5 administrative districts and 45 Blocks (sub-district) as shown in Figures 3 and 4 respectively. In Table 1 the physical area of the blocks/districts has been provided. -

Talcher-Dhamra Stretch of Rivers, Geonkhali-Charbatia Stretch of East Coast Canal, Charbatia-Dhamra Stretch of Matai River and Mahanadi Delta Rivers) Act, 2008 Act No
THE NATIONAL WATERWAY (TALCHER-DHAMRA STRETCH OF RIVERS, GEONKHALI-CHARBATIA STRETCH OF EAST COAST CANAL, CHARBATIA-DHAMRA STRETCH OF MATAI RIVER AND MAHANADI DELTA RIVERS) ACT, 2008 ACT NO. 23 OF 2008 [17th November, 2008.] An Act to provide for the declaration of the Talcher-Dhamra stretch of Brahmani-Kharsua- Dhamra rivers, Geonkhali-Charbatia stretch of East Coast Canal, Charbatia-Dhamra stretch of Matai river and Mahanadi delta rivers between Mangalgadi and Paradip in the States of West Bengal and Orissa to be a national waterway and also to provide for the regulation and development of the said stretch of the rivers and the canals for the purposes of shipping and navigation on the said waterway and for matters connected therewith or incidental thereto. BE it enacted by Parliament in the Fifty-ninth Year of the Republic of India as follows:— 1. Short title and commencement.—(1) This Act may be called the National Waterway (Talcher-Dhamra Stretch of Rivers, Geonkhali-Charbatia Stretch of East Coast Canal, Charbatia- Dhamra Stretch of Matai River and Mahanadi Delta Rivers) Act, 2008. (2) It shall come into force on such date1 as the Central Government may, by notification in the Official Gazette, appoint. 2. Declaration of certain stretches of rivers and canals as National Waterway.—The Talcher- Dhamra stretch of Brahmani-Kharsua-Dhamra rivers, Geonkhali-Charbatia stretch of East Coast Canal, Charbatia-Dhamra stretch of Matai river and Mahanadi delta rivers between Mangalgadi and Paradip, the limits of which are specified in the Schedule, is hereby declared to be a National Waterway. 3. -

Research Setting
S.K. Acharya, G.C. Mishra and Karma P. Kaleon Chapter–6 Research Setting Anshuman Jena, S K Acharya, G.C. Mishra and Lalu Das In any social science research, it is hardly possible to conceptualize and perceive the data and interpret the data more accurately until and unless a clear understanding of the characteristics in the area and attitude or behavior of people is at commend of the interpreter who intends to unveil an understanding of the implications and behavioral complexes of the individuals who live in the area under reference and from a representative part of the larger community. The socio demographic background of the local people in a rural setting has been critically administered in this chapter. A research setting is a surrounding in which inputs and elements of research are contextually imbibed, interactive and mutually contributive to the system performance. Research setting is immensely important in the sense because it is characterizing and influencing the interplays of different factors and components. Thus, a study on Perception of Farmer about the issues of Persuasive certainly demands a local unique with natural set up, demography, crop ecology, institutional set up and other socio cultural Social Ecology, Climate Change and, The Coastal Ecosystem ISBN: 978-93-85822-01-8 149 Anshuman Jena, S K Acharya, G.C. Mishra and Lalu Das milieus. It comprises of two types of research setting viz. Macro research setting and Micro research setting. Macro research setting encompasses the state as a whole, whereas micro research setting starts off from the boundaries of the chosen districts to the block or village periphery. -

Influence of Water Quality on the Biodiversity of Phytoplankton in Dhamra River Estuary of Odisha Coast, Bay of Bengal
March JASEM ISSN 1119-8362 Full-text Available Online at J. Appl. Sci. Environ. Manage. , 2011 All rights reserved www.bioline.org.br/ja Vol. 15 (1) 69 - 74 Influence of Water quality on the biodiversity of phytoplankton in Dhamra River Estuary of Odisha Coast, Bay of Bengal PALLEYI, S; KAR, R N; *PANDA, C R Institute of Minerals & Materials Technology, Bhubaneswar-751013, India *Corresponding author : [email protected] ABSTRACT: Dhamra estuarine ecosystem is a hotspot of rich biological diversity which supports a patch of mangrove along with unique flora and fauna. In this study, the diversity of phytoplankton population and other factors that control their growth and biodiversity were investigated. The samples were collected monthly from Dhamra estuary of Bay of Bengal at 6 different stations (grouped under three regions) from March -2008 to February -2009. A total of 41 genera of phytoplankton species belonging to 4 classes of algae were identified. The maximum value of 9.3 X 10 4 cells l -1 was recorded in post monsoon season. Phytoplankton of Bacillariophyceae, appearing throughout the year, and represent majority of population (75-94%) at all the sampling stations, followed by Dinophyceae (3-14%), Cyanophyceae (3-8%) and Chlorophyceae (0-4%) classes. The Shannon- weavers diversity index (H) remains between 0.22 and 2.49. Based on the correlation coefficient data, phytoplankton shows positive relationship with DO, salinity, nutrients and negative relationship with temperature and turbidity. Present study shows that the occurrence and diversity of these primary producers do not subscribe to a single dimensional phenomenon of a single factor, rather than, a consequence of a series of supported factors which will help to maintain and balance such type of fragile ecosystem. -

A Note from WIO on the Rengali Dam and Flood Management
Special Note – II on ‘Odisha Floods 2011’ from Water Initiatives Odisha (WIO) 24th September, 2011 Different Designs, Same Management ‘Did we manage the Rengali dam the way we managed Hirakud?’ asks Water Initiatives Odisha, as it brings a special note on this issue in response to the latest spell of the flood disaster in northern Odisha Rivers. The latest spectre of floods over Brahmani and Baitarani has once again brought the Dam Operations and Management systems of the state into question. This time the focus goes to the Rengali dam. Before going further to the discussion, we would like to bring to your notice the water storage level that was maintained in the Rengali dam on select dates as against the recommended Rule Curve for that dam. Select Date Recommended Maximum Level at which the Reservoir Reservoir and Minimum was kept Limits (by Rule Curve) 1st July 109.72 M / 109.72 M 114.84 M 1st August 115.85 M/ 115.85 M 114.91 M 1st September 122.50 M/ 121.95 M 122.36 M 7th September 122.50 M/ 121.95 M 122.87 M 14th September 122.50 M/ 121.95 M 123.54 M 20th September 122.50 M/ 121.95 M 123.66 M 21st September 122.50 M/ 121.95 M 123.55 M 22nd September 122.50 M/ 121.95 M 123.56 M 23rd September 122.50 M/ 121.95 M 123.56 M 24th September 122.50 M/ 121.95 M 124.39 M As can be observed from the above, even as warnings of depression/low pressure were coming in (and in fact that has historically brought rain at this time of the year over Brahmani and Baitarani river basins), the Reservoir was kept consistently at a higher level. -

Migration As an Adaptation to Climate Change in Mahanadi Delta
Migration as an Adaptation to Climate Change in Mahanadi Delta Shouvik Das, Sugata Hazra* , Tuhin Ghosh*, Somnath Hazra, and Amit Ghosh (*Presenting Authors) School of Oceanographic Studies, Jadavpur University, India Abstract Number: ABSSUB-989 Adaptation Future 2016, Rotterdam, Netherlands Introduction Study Area Socio-Economic Profile • Agriculture and fishery sectors of natural resource based The Decadal Variation in Population Since 1901 Map of India economy of deltas are increasingly becoming unprofitable due to 2,500,000 Climate Change. Bhadrak 2,000,000 Kendrapara • This results in large scale labour migration, in absence of Jagatsinghapur 1,500,000 alternative livelihood option in the Mahanadi delta, Odisha, Mahanadi Delta Khordha Odisha: 270 persons per sq. km. 1,000,000 Puri India: 382 persons per sq. km. India. Population Total • Labour migration increased manifold in the coastal region of 500,000 Odisha in the aftermath of super cyclones of 1999 and 2013. - 1901 1911 1921 1931 1941 1951 Year 1961 1971 1981 1991 2001 2011 • The present research discusses whether migration can be 30 Population Growth Rate (%), 2001-2011 considered as an adaptation option when the mainstay of 20 Odisha: 14.05% livelihood, i.e. agriculture is threatened by repeated flooding, sea 10 0 level rise, cyclone and storm surges, salinization of soil and crop (%) Rate Growth Bhadrak Kendrapara Jagatsinghapur Khordha Puri 1% failure due to temperature stress imposed by climate change. 5% Malkangiri 205 9% Koraput 170 26% 157 5% Methodology Rayagada 146 -

Information( 13 Tahasil & 80 R.I. Offices)
Information( 13 Tahasil & 80 R.I. Offices) CHAMPUA TAHASIL Champua Tahasil started functioning w.e.f. 01.04.1963. The total area of this Tahasil is Ac. 84,754.274. (342.98 SQ. KMS.) This Tahasil is coming under the jurisdiction of Champua Sub- Division and the area of this Tahasil is co-terminus with the area of Champua Block and Champua NAc consisting six R.I. circles namely Champua, Jyotipur, Chimila, Remuuli, Karanjia and Bhanda. This Tahasil is constituted with 151 revenue villages under 23 G.Ps. and newly created Champua NAC which coveres three police stations namely Champua, Joda (part) and Baria(part) . The total population of the Tahasil is 1, 15,103 as per 2011 census. Communication facilities are available from Tahasil head quarter to District head quarter. The principal income source of the people is cultivation and business. Champua Tahasil is situated 50 Kms away from District Headquarters. This Tahasil is surrounded by Maurbhanj District in east, Barbil Tahsil in west, Singhbhum District of Jharkhand State in north and Jhumpura Tahasil in south. Brief notes on R.I. Circles Under Champua Tahasil Champua : This R.I. circle is consisting of 17 revenue villages covering Rajia G.P. and newly declared Champua NAC having area of Ac.11,967.650. All the 17 villages are coming under jurisdiction of Champua P.S. and co-terminus with the area of Rajia G.P. and Champua NAC. Chimila : This R.I. circle is consisting of 27 villages covering Kodagadia, Jamudalak, Sunaposi and Kutariposi G.Ps. having area of Ac.14,484.513. -
![DISASTER MANAGEMENT PLAN. [Dowr] ****************************************** 1](https://docslib.b-cdn.net/cover/6501/disaster-management-plan-dowr-1-1816501.webp)
DISASTER MANAGEMENT PLAN. [Dowr] ****************************************** 1
DISASTER MANAGEMENT PLAN. [DoWR] ****************************************** 1. Introduction The state Odisha is ranked as the 5th most flood prone state of the country after UP, Bihar, Assam and West Bengal with a flood prone area of 33400 km2. The south-west monsoon brings rains to the state from June to September every year. The state receives an average annual rainfall of 1500 mm and more than 80% of it occurs during monsoon period only. The coastal districts of the state are more vulnerable to frequent low pressure, cyclonic storms, depression and deep depression. The state has five major river basins namely Mahanadi, Brahmani, Baitarani, Subarnarekha and Rushikulya which cause high floods in their respective deltas. The rivers like Vamshadhara and Burhabalang also cause flash floods due to instant runoff from their hilly catchment. It is a fact that the three major river system Mahanadi, Brahmani and Baitarani forms a single delta during high flood and in most of the cases the flood water of these three systems blend together causing considerable flood havoc. Besides the state has 476.40 kms of coastline on the west of Bay of Bengal. The flood problem becomes more severe when the flood synchronies with high tides causing slow recede of flood. The silt deposited constantly by the waves in the delta area raises the flood level and the rivers often overflow their banks. The flood problem in the state generally aggravated due to some or all of the reasons as below: - Erratic monsoon, heavy monsoon rainfall accompanied by low pressures, depressions, deep depressions and cyclones. - Dam releases due to heavy inflows, thus causing massive outflows in the river. -

SGAT Bulletin June 2015
ISSN 0972-2173 RNI Regd. No: ORIENG/2000/2296 Vol. 16 • June 2015 • No. 1 EDITORIAL BOARD EXECUTIVE COUNCIL MEMBERS (2013-2015 term) EDITOR President Dr. S.K. Sarangi Dr. S. K. Sarangi 102, Royale Residency Vice-Presidents Plot No. VIP -48, IRC Village, Prof. M.C. Dash Bhubaneswar – 751 015 Prof. Omkar Nath Mohanty Phone: 0674-2551080 General Secretary (I/c) Fax: 0674-2551687 Shri Sujit Kumar Mohanty E-mail: [email protected] Jt. Secretaries [email protected] Dr. S.C. Mahala Shri Sujit Kumar Mohanty ADVISORS MEMBERS Treasurer Shri T. Mohanta Prof. Dr. S. Acharya Dr. More Ramulu Members Chairman Principal Scientist Shri Nalini Ranjan Patnaik 155, VIP Colony CSIR-Central Institute of Dr. B. M. Faruque Bhubaneswar – 751 015 Mining & Fuel Research Shri Rajib Lochan Mohanty Nagpur - 440006 Shri Jiban Mohapatra Dr. Rabindra Nath Hota Prof. Dr. Madhab Ch. Dash Prof. Dr. R.N. Hota Dr. R.C. Mohanty 101, Ananda Villa P.G. Dept. of Geology Shri R.N. Patra 45, VIP Area, IRC Village Utkal University Dr. Subhransu Bhusan Ray Bhubaneswar 751015 Vani Vihar, Bhubaneswar Shri Ardhendu Mohapatra Shri Subhransu Mishra Shri K.C. Pradhan Shri S.N. Padhi Dr. B.K. Mohapatra Shri M.V. Rao A/8, Palaspalli Former Scientist Shri G.C. Das B.D.A. Complex Institute of Minerals and Mrs. Nirupama Das Bhubaneswar – 751 020 Materials Technology Shri S.K. Das Bhubaneswar – 751 013 Shri Pravakar Rout Shri J. K. Hota Shri G.S. Khuntia Dr. S.K. Biswal Shri Sudhakara Adhikaree Shri Sanjay Patnaik Shri Alok Behari Panigrahi Shri Lalit Kumar Tewari, IFS Publication of the Society of Geoscientists and Allied Technologists Complementary for Members of the Society The statements made or views expressed in articles in this publication do not necessarily reflect the opinion of SGAT SGAT Bulletin Vol. -
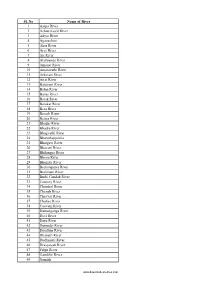
List of Rivers in India
Sl. No Name of River 1 Aarpa River 2 Achan Kovil River 3 Adyar River 4 Aganashini 5 Ahar River 6 Ajay River 7 Aji River 8 Alaknanda River 9 Amanat River 10 Amaravathi River 11 Arkavati River 12 Atrai River 13 Baitarani River 14 Balan River 15 Banas River 16 Barak River 17 Barakar River 18 Beas River 19 Berach River 20 Betwa River 21 Bhadar River 22 Bhadra River 23 Bhagirathi River 24 Bharathappuzha 25 Bhargavi River 26 Bhavani River 27 Bhilangna River 28 Bhima River 29 Bhugdoi River 30 Brahmaputra River 31 Brahmani River 32 Burhi Gandak River 33 Cauvery River 34 Chambal River 35 Chenab River 36 Cheyyar River 37 Chaliya River 38 Coovum River 39 Damanganga River 40 Devi River 41 Daya River 42 Damodar River 43 Doodhna River 44 Dhansiri River 45 Dudhimati River 46 Dravyavati River 47 Falgu River 48 Gambhir River 49 Gandak www.downloadexcelfiles.com 50 Ganges River 51 Ganges River 52 Gayathripuzha 53 Ghaggar River 54 Ghaghara River 55 Ghataprabha 56 Girija River 57 Girna River 58 Godavari River 59 Gomti River 60 Gunjavni River 61 Halali River 62 Hoogli River 63 Hindon River 64 gursuti river 65 IB River 66 Indus River 67 Indravati River 68 Indrayani River 69 Jaldhaka 70 Jhelum River 71 Jayamangali River 72 Jambhira River 73 Kabini River 74 Kadalundi River 75 Kaagini River 76 Kali River- Gujarat 77 Kali River- Karnataka 78 Kali River- Uttarakhand 79 Kali River- Uttar Pradesh 80 Kali Sindh River 81 Kaliasote River 82 Karmanasha 83 Karban River 84 Kallada River 85 Kallayi River 86 Kalpathipuzha 87 Kameng River 88 Kanhan River 89 Kamla River 90 -

Rivers of India
Downloaded From examtrix.com Compilation of Rivers www.onlyias.in Mahanadi RiverDownloaded From examtrix.com Source: Danadkarnya Left bank: Sheonath, Hasdo and Mand Right bank: Tel, Jonk, Ong Hirakund dam Olive Ridley Turtles: Gahirmatha beach, Orissa: Nesting turtles River flows through the states of Chhattisgarh and Odisha. River Ends in Bay of Bengal Mahanadi RiverDownloaded From examtrix.com Mahanadi RiverDownloaded From examtrix.com • The Mahanadi basin extends over states of Chhattisgarh and Odisha and comparatively smaller portions of Jharkhand, Maharashtra and Madhya Pradesh, draining an area of 1.4 lakh Sq.km. • It is bounded by the Central India hills on the north, by the Eastern Ghats on the south and east and by the Maikala range on the west. • The Mahanadi (“Great River”) follows a total course of 560 miles (900 km). • It has its source in the northern foothills of Dandakaranya in Raipur District of Chhattisgarh at an elevation of 442 m. • The Mahanadi is one of the major rivers of the peninsular rivers, in water potential and flood producing capacity, it ranks second to the Godavari. Mahanadi RiverDownloaded From examtrix.com • Other small streams between the Mahanadi and the Rushikulya draining directly into the Chilka Lake also forms the part of the basin. • After receiving the Seonath River, it turns east and enters Odisha state. • At Sambalpur the Hirakud Dam (one of the largest dams in India) on the river has formed a man-made lake 35 miles (55 km) long. • It enters the Odisha plains near Cuttack and enters the Bay of Bengal at False Point by several channels. -

Infrastructure Study Report for 300 Mt Steel by 2025
DRAFT INFRASTRUCTURE STUDY REPORT FOR 300 MT STEEL BY 2025 MECON LIMITED RANCHI- 834002 JULY, 2014 (R0) No. 11.14.2014.PP 2151 JUNE, 2015 (R1) DRAFT JOINT PLANT COMMITTEE Ministry of Steel, GOI INFRASTRUCTURE STUDY REPORT FOR 300 MT STEEL BY 2025 MECON LIMITED Ranchi – 834002 No. : 11.14.2014.PP 2151 JULY , 2014 (R0) JUNE, 2015 (R1) INFRASTRUCTURE STUDY REPORT FOR 300 MT STEEL BY 2025 GOVT. OF INDIA, MINISTRY OF STEEL PREFACE It is largely being felt now by Country’s policy makers that manufacturing has to be the backbone of future growth strategy of India over the next decade. Accordingly, the new manufacturing policy aims at increasing manufacturing growth rate to 11-12% by 2016-17 and raising its share in GDP from current 16% to 25% by 2025. The policy envisages creation of National Investment & Manufacturing Zones (NIMZs) equipped with world class infrastructure facilities to promote manufacturing activities in the country. To achieve the manufacturing growth of GDP’s share from 16% to 25% by 2025, there will be substantial increase in steel demand. Some of the NMIZs are being planned in mineral rich states offering excellent potential location for setting up new steel plants. Draft National Steel Policy 2012 targets crude steel capacity of 300 Mt in the country by the middle of the next decade (2025-26). A High Level Committee on Manufacturing (HLCM) in its meeting held on 9th July 2013 which was chaired by the then Hon’ble Prime Minister endorsed the growth strategy targeting National Mission of 300 Mt crude steel output by 2025-26.