Microarchitecture of the Dyad
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Unnatural Verticilide Enantiomer Inhibits Type 2 Ryanodine Receptor-Mediated Calcium Leak and Is Antiarrhythmic
Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic Suzanne M. Batistea,1, Daniel J. Blackwellb,1, Kyungsoo Kimb,1, Dmytro O. Kryshtalb, Nieves Gomez-Hurtadob, Robyn T. Rebbeckc, Razvan L. Corneac, Jeffrey N. Johnstona,2, and Bjorn C. Knollmannb,2 aDepartment of Chemistry, Vanderbilt University, Nashville, TN 37235; bDepartment of Medicine, Vanderbilt University Medical Center, Nashville, TN 37232; and cDepartment of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN 55455 Edited by Dale L. Boger, The Scripps Research Institute, La Jolla, CA, and approved January 15, 2019 (received for review September 27, 2018) Ca2+ leak via ryanodine receptor type 2 (RyR2) can cause poten- heart diseases associated with both atrial and ventricular arrhyth- tially fatal arrhythmias in a variety of heart diseases and has also mia (9). Mutations in RyR2 and its binding partners, which increase + been implicated in neurodegenerative and seizure disorders, mak- SR Ca2 leak, cause primary atrial and ventricular arrhythmia ing RyR2 an attractive therapeutic target for drug development. syndromes such as catecholaminergic polymorphic ventricular Here we synthesized and investigated the fungal natural product tachycardia (CPVT), providing strong evidence for the mechanistic and known insect RyR antagonist (−)-verticilide and several conge- contribution of RyR2 to arrhythmia risk in humans (10). Further ners to determine their activity against mammalian RyR2. Although support comes from gene-targeted mouse models of CPVT, where + the cyclooligomeric depsipeptide natural product (−)-verticilide had catecholamine-induced spontaneous Ca2 release from the SR no effect, its nonnatural enantiomer [ent-(+)-verticilide] signifi- via RyR2 generates potentially fatal cardiac arrhythmias (11, 12). -

Calcium-Induced Calcium Release in Smooth Muscle7 Loose Coupling
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Calcium-induced Calcium Release in Smooth Muscle✪ Loose Coupling between the Action Potential and Calcium Release M.L. Collier, G. Ji, Y.-X. Wang, and M.I. Kotlikoff From the Department of Animal Biology, School of Veterinary Medicine, University of Pennsylvania, Philadelphia, Pennsylvania 19104-6046 abstract Calcium-induced calcium release (CICR) has been observed in cardiac myocytes as elementary cal- cium release events (calcium sparks) associated with the opening of L-type Ca2ϩ channels. In heart cells, a tight coupling between the gating of single L-type Ca2ϩ channels and ryanodine receptors (RYRs) underlies calcium re- lease. Here we demonstrate that L-type Ca2ϩ channels activate RYRs to produce CICR in smooth muscle cells in the form of Ca2ϩ sparks and propagated Ca2ϩ waves. However, unlike CICR in cardiac muscle, RYR channel open- ing is not tightly linked to the gating of L-type Ca2ϩ channels. L-type Ca2ϩ channels can open without triggering Ca2ϩ sparks and triggered Ca2ϩ sparks are often observed after channel closure. CICR is a function of the net flux of Ca2ϩ ions into the cytosol, rather than the single channel amplitude of L-type Ca2ϩ channels. Moreover, unlike CICR in striated muscle, calcium release is completely eliminated by cytosolic calcium buffering. Thus, L-type Ca2ϩ channels are loosely coupled to RYR through an increase in global [Ca2ϩ] due to an increase in the effective distance between L-type Ca2ϩ channels and RYR, resulting in an uncoupling of the obligate relationship that exists in striated muscle between the action potential and calcium release. -

Surface Glycoproteomic Analysis of Hepatocellular Carcinoma Cells by Affinity Enrichment and Mass Spectrometric Identification
Glycoconj J (2012) 29:411–424 DOI 10.1007/s10719-012-9420-3 Surface glycoproteomic analysis of hepatocellular carcinoma cells by affinity enrichment and mass spectrometric identification Wei Mi & Wei Jia & Zhaobin Zheng & Jinglan Wang & Yun Cai & Wantao Ying & Xiaohong Qian Received: 14 April 2012 /Revised: 5 June 2012 /Accepted: 12 June 2012 /Published online: 1 July 2012 # Springer Science+Business Media, LLC 2012 Abstract Cell surface glycoproteins are one of the most surface-capturing (CSC) technique was an approach specif- frequently observed phenomena correlated with malignant ically targeted at membrane glycoproteins involving the growth. Hepatocellular carcinoma (HCC) is one of the most affinity capture of membrane glycoproteins using glycan malignant tumors in the world. The majority of hepatocel- biotinylation labeling on intact cell surfaces. To characterize lular carcinoma cell surface proteins are modified by glyco- the cell surface glycoproteome and probe the mechanism of sylation in the process of tumor invasion and metastasis. tumor invasion and metastasis of HCC, we have modified Therefore, characterization of cell surface glycoproteins can and evaluated the cell surface-capturing strategy, and ap- provide important information for diagnosis and treatment plied it for surface glycoproteomic analysis of hepatocellu- of liver cancer, and also represent a promising source of lar carcinoma cells. In total, 119 glycosylation sites on 116 potential diagnostic biomarkers and therapeutic targets for unique glycopeptides were identified, corresponding to 79 hepatocellular carcinoma. However, cell surface glycopro- different protein species. Of these, 65 (54.6 %) new pre- teins of HCC have been seldom identified by proteomics dicted glycosylation sites were identified that had not pre- approaches because of their hydrophobic nature, poor solu- viously been determined experimentally. -

Absence of Triadin, a Protein of the Calcium Release Complex, Is Responsible for Cardiac Arrhythmia with Sudden Death in Human
Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Nathalie Roux-Buisson, Marine Cacheux, Anne Fourest-Lieuvin, J. Fauconnier, Julie Brocard, Isabelle Denjoy, Philippe Durand, Pascale Guicheney, Florence Kyndt, Antoine Leenhardt, et al. To cite this version: Nathalie Roux-Buisson, Marine Cacheux, Anne Fourest-Lieuvin, J. Fauconnier, Julie Brocard, et al.. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human.. Human Molecular Genetics, Oxford University Press (OUP), 2012, 21 (12), pp.2759-67. 10.1093/hmg/dds104. inserm-00763211 HAL Id: inserm-00763211 https://www.hal.inserm.fr/inserm-00763211 Submitted on 10 Dec 2012 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. HMG Advance Access published March 29, 2012 Human Molecular Genetics, 2012 1–9 doi:10.1093/hmg/dds104 Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human Nathalie Roux-Buisson1,2,3,4,{, Marine Cacheux1,4,{, Anne Fourest-Lieuvin1,4,5, Jeremy Fauconnier6,7,8,9, Julie Brocard1,4, Isabelle Denjoy10, Philippe Durand11, Pascale Guicheney12,13, Florence Kyndt14,15,16, Antoine Leenhardt10, Herve´ Le Marec14,16,17, Vincent Lucet18, Philippe Mabo19, Vincent Probst14,16,17, Nicole Monnier1,2, Pierre F. -

Triadin, a Linker for Calsequestrin and the Ryanodine Receptor
Triadin, a Linker for Calsequestrin and the Ryanodine Receptor Wei Guo,* Annelise 0. Jorgensen,' and Kevin P. Campbell* *Howard Hughes Medical Institute, Department of Physiology and Biophysics, University of Iowa College of Medicine, Iowa City, Iowa 52242, and *Departmentof Anatomy and Cell Biology, University of Toronto, Toronto, Ontario, Canada M5S 1A8 Introduction Protein components of the triad junction play essential roles in muscle excitation- contraction coupling (EC coupling). Considerable research has been performed on the identification and characterization of proteins that regulate calcium storage and release from the sarcoplasmic reticulum (McPherson and Campbell, 1993; Franzini- Armstrong and Jorgensen, 1994). Key proteins characterized include the dihydro- pyridine receptor; the voltage sensor and L-type calcium channel in t-tubules; the ryanodine receptor/Ca2+-releasechannel in the terminal cisternae of the sarcoplas- mic reticulum; and calsequestrin, a moderate-affinity, high-capacity calcium-bind- ing protein located in the lumen of the junctional sarcoplasmic reticulum. Study of these proteins has been instrumental to our understanding of the molecular mecha- nisms of EC coupling. Recent research from our laboratory has focused on triadin, an abundant transmembrane protein in the junctional sarcoplasmic reticulum. Here, we briefly review recent results on the structure of triadin and its interactions with other protein components of the junctional complex in skeletal and cardiac muscle. Identification of Triadin Using purified skeletal muscle triads, we generated a library of monoclonal anti- bodies against different proteins of the junctional sarcoplasmic reticulum (Camp- bell et al., 1987). Several monoclonal antibodies recognize a protein of 94 kD (now called triadin) on reducing SDS-PAGE (Fig. -

Dual Copy Number Variants Involving 16P11 and 6Q22 in a Case
European Journal of Human Genetics (2013) 21, 361–366 & 2013 Macmillan Publishers Limited All rights reserved 1018-4813/13 www.nature.com/ejhg LETTERS 32 duplications, 0.78%). A detailed review of 18 patients found Dual copy number variants that the most consistent clinical manifestations in these individuals were intellectual impairment and speech and language delays.8 involving 16p11 and These findings were supported by a similar study that included 7400 patients who had undergone array comparative genomic 6q22inacaseof hybridisation (array-CGH) testing in a clinical context, 45 of whom carried 16p11.2 anomalies (27 deletions, 18 duplications, 0.6%).3 childhood apraxia of Phenotypic characterisation of 27 individuals also found that all had speech and language delays and cognitive impairment.3 Other predomi- nant features of 16p11.2 syndrome include dysmorphism, macrocephaly speech and pervasive and autistic disorders.3,4,8,14 However, all of these features have been disputed and it is likely that ascertainment bias will affect the developmental disorder conclusions of many studies, particularly those that focus upon single cases. Thus, the characterisation of the relationships between genetic aberration and clinical presentation is ongoing and will require European Journal of Human Genetics (2013) 21, 361–365; further, more refined, studies with detailed investigations of this doi:10.1038/ejhg.2012.166; published online 22 August 2012 chromosome region and consistent phenotyping of affected individuals. The child described here was originally assessed for the presence of In this issue, Raca et al1 present two cases of childhood apraxia of FOXP2 (OMIM #605317) mutations and rearrangements, as part of 15 speech (CAS) arising from microdeletions of chromosome 16p11.2. -

Calcium Signaling and Cardiac Arrhythmias
Review Calcium Signaling Series Donald M. Bers, Guest Editor Calcium Signaling and Cardiac Arrhythmias Andrew P. Landstrom, Dobromir Dobrev, Xander H.T. Wehrens Abstract: There has been a significant progress in our understanding of the molecular mechanisms by which calcium (Ca2+) ions mediate various types of cardiac arrhythmias. A growing list of inherited gene defects can cause potentially lethal cardiac arrhythmia syndromes, including catecholaminergic polymorphic ventricular Downloaded from tachycardia, congenital long QT syndrome, and hypertrophic cardiomyopathy. In addition, acquired deficits of multiple Ca2+-handling proteins can contribute to the pathogenesis of arrhythmias in patients with various types of heart disease. In this review article, we will first review the key role of Ca2+ in normal cardiac function—in particular, excitation–contraction coupling and normal electric rhythms. The functional involvement of Ca2+ in distinct arrhythmia mechanisms will be discussed, followed by various inherited arrhythmia syndromes caused 2+ http://circres.ahajournals.org/ by mutations in Ca -handling proteins. Finally, we will discuss how changes in the expression of regulation of Ca2+ channels and transporters can cause acquired arrhythmias, and how these mechanisms might be targeted for therapeutic purposes. (Circ Res. 2017;120:1969-1993. DOI: 10.1161/CIRCRESAHA.117.310083.) Key Words: arrhythmias, cardiac ■ atrial fibrillation ■ calcium channels ■ cardiomyopathy ■ ryanodine receptor calcium release channel 2+ by guest on June 11, 2017 he bivalent cation calcium (Ca ) represents one of the Overview of Excitation–Contraction Tmost ubiquitous signal transduction molecules known.1 Coupling in the Heart It mediates a diverse array of biological functions including Regular contraction of the heart requires the conversion of muscle contraction, cellular exocytosis, neuronal activity, and electric activation (excitation) into mechanical force (con- triggering of programmed cell death. -

Generation of a Triadin Knockout Syndrome Zebrafish Model
International Journal of Molecular Sciences Article Generation of a Triadin KnockOut Syndrome Zebrafish Model Vanilla Martina Vecchi 1,†, Marco Spreafico 1,† , Alessia Brix 1, Anna Santoni 2, Simone Sala 3 , Anna Pistocchi 1,* , Anna Marozzi 1,† and Chiara Di Resta 2,† 1 Department of Medical Biotechnology and Translational Medicine, Università degli Studi di Milano, LITA, Segrate, 20090 Milan, Italy; [email protected] (V.M.V.); marco.spreafi[email protected] (M.S.); [email protected] (A.B.); [email protected] (A.M.) 2 UOC Clinical Genomics, IRCCS San Raffaele Hospital, 20132 Milan, Italy; [email protected] (A.S.); [email protected] (C.D.R.) 3 Department of Cardiac Electrophysiology and Arrhythmology, IRCCS San Raffaele Hospital, 20132 Milan, Italy; [email protected] * Correspondence: [email protected] † These authors contributed equally to the work. Abstract: Different forms of sudden cardiac death have been described, including a recently identified form of genetic arrhythmogenic disorder, named “Triadin KnockOut Syndrome” (TKOS). TKOS is associated with recessive mutations in the TRDN gene, encoding for TRIADIN, but the pathogenic mechanism underlying the malignant phenotype has yet to be completely defined. Moreover, patients with TKOS are often refractory to conventional treatment, substantiating the need to identify new therapeutic strategies in order to prevent or treat cardiac events. The zebrafish (Danio rerio) heart is highly comparable to the human heart in terms of functions, signal pathways and ion channels, representing a good model to study cardiac disorders. In this work, we generated the first zebrafish model for trdn loss-of-function, by means of trdn morpholino injections, and characterized Citation: Vecchi, V.M.; Spreafico, M.; its phenotype. -
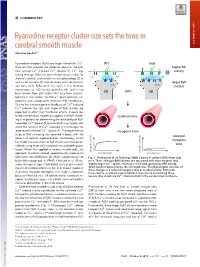
Ryanodine Receptor Cluster Size Sets the Tone in Cerebral Smooth Muscle
COMMENTARY Ryanodine receptor cluster size sets the tone in cerebral smooth muscle COMMENTARY Christian Soellera,1 + Ryanodine receptors (RyRs) are large intracellular Ca2 WT mdx channels that provide the molecular basis of the pro- K+ K+ higher BK + + + cess termed Ca2 -induced Ca2 release (1). Ca2 sig- activity naling through RyRs has been shown to be critical for CaC BK BK skeletal, cardiac, and smooth muscle physiology (2) as well as for neurons (3) and secretory cells like pancre- larger RyR atic beta cells. RyRs were first seen in the electron RyRs RyRs clusters microscope as ∼30-nm-size particles (4), and it had SMCs been known from EM studies that they form clusters, SR SR typically in the narrow “junctional” space between sar- colemma and sarcoplasmic reticulum (SR) membranes. + Due to the inherent positive feedback of Ca2 -induced + Ca2 release, the size and shape of RyR clusters are expected to affect their functional activity. Indeed, de- SMCs SMCs tailed mathematical modeling suggests that RyR cluster- cerebral artery ing is important for determining the excitability of RyR- + mediated Ca2 release (5, 6) and could, in principle, also + affect the amount of Ca2 released in microscopic re- 2+ lease events termed Ca sparks (7). The experimental myogenic tone study of RyR clustering has received a boost with the reduced advent of optical superresolution microscopy, which myogenic has made the assessment of RyR cluster size more acces- sible by using essentially standard immunolabeling pro- tone tocols. When first applied in cardiac muscle cells, this myogenic tone (%) tone myogenic approach revealed a broad, approximately exponential vessel pressure (%) tone myogenic vessel pressure RyR cluster size distribution (8), which had previously not Fig. -

Molecular Genetics of Exercise-Induced
European Journal of Human Genetics (2003) 11, 888–891 & 2003 Nature Publishing Group All rights reserved 1018-4813/03 $25.00 www.nature.com/ejhg SHORT REPORT Molecular genetics of exercise-induced polymorphic ventricular tachycardia: identification of three novel cardiac ryanodine receptor mutations and two common calsequestrin 2 amino-acid polymorphisms Pa¨ivi J Laitinen1, Heikki Swan2 and Kimmo Kontula*,1 1Department of Medicine and Biomedicum Helsinki, Helsinki, Finland; 2Department of Cardiology, University of Helsinki, Helsinki, Finland Mutations of two myocardial calcium signaling molecules, ryanodine receptor 2 (RYR2) and calsequestrin 2 (CASQ2), may cause catecholaminergic polymorphic ventricular tachycardia (CPVT), a severe inherited arrhythmic disease manifesting with salvoes of exercise-induced bidirectional and polymorphic tachycardias. We screened 12 Finnish CPVT probands for mutations in these genes and identified three novel RYR2 mutations (V2306I, P4902L, R4959Q), which were absent in unaffected and control individuals. Although no obvious disease-causing mutations were identified in the CASQ2 gene, the molecular screening revealed two novel amino-acid polymorphisms (T66A and V76M). The frequencies of these polymorphisms in 185 unrelated probands with long QT syndrome and in 280 healthy blood donors were not significantly different. These data, combined with our previous findings, show that RYR2 mutations are present in at least 6/16 (38%) of the catecholaminergic polymorphic ventricular tachycardia families, while CASQ2 -

An Investigative Mathematical Model of Calcium-Induced Calcium Release
Biophysical Journal Volume 83 July 2002 59–78 59 Termination of Cardiac Ca2؉ Sparks: An Investigative Mathematical Model of Calcium-Induced Calcium Release Eric A. Sobie,* Keith W. Dilly,* Jader dos Santos Cruz,* W. Jonathan Lederer,* and M. Saleet Jafri† *Medical Biotechnology Center, University of Maryland Biotechnology Center, Baltimore, Maryland 21201, and †Department of Mathematical Sciences, The University of Texas at Dallas, Richardson, Texas 75083 USA ABSTRACT A Ca2ϩ spark arises when a cluster of sarcoplasmic reticulum (SR) channels (ryanodine receptors or RyRs) opens to release calcium in a locally regenerative manner. Normally triggered by Ca2ϩ influx across the sarcolemmal or transverse tubule membrane neighboring the cluster, the Ca2ϩ spark has been shown to be the elementary Ca2ϩ signaling event of excitation–contraction coupling in heart muscle. However, the question of how the Ca2ϩ spark terminates remains a central, unresolved issue. Here we present a new model, “sticky cluster,” of SR Ca2ϩ release that simulates Ca2ϩ spark behavior and enables robust Ca2ϩ spark termination. Two newly documented features of RyR behavior have been incorpo- rated in this otherwise simple model: “coupled gating” and an opening rate that depends on SR lumenal [Ca2ϩ]. Using a Monte Carlo method, local Ca2ϩ-induced Ca2ϩ release from clusters containing between 10 and 100 RyRs is modeled. After release is triggered, Ca2ϩ flux from RyRs diffuses into the cytosol and binds to intracellular buffers and the fluorescent Ca2ϩ indicator fluo-3 to produce the model Ca2ϩ spark. Ca2ϩ sparks generated by the sticky cluster model resemble those observed experimentally, and Ca2ϩ spark duration and amplitude are largely insensitive to the number of RyRs in a cluster. -

Defects in T-Tubular Electrical Activity Underlie Local Alterations of Calcium Release in Heart Failure
Defects in T-tubular electrical activity underlie local alterations of calcium release in heart failure Claudia Crocinia, Raffaele Coppinib, Cecilia Ferrantinic, Ping Yand, Leslie M. Loewd, Chiara Tesic, Elisabetta Cerbaib, Corrado Poggesic, Francesco S. Pavonea,e,f, and Leonardo Sacconia,f,1 aEuropean Laboratory for Non-Linear Spectroscopy, 50019 Florence, Italy; bDivision of Pharmacology, Department “NeuroFarBa,” University of Florence, 50139 Florence, Italy; cDivision of Physiology, Department of Experimental and Clinical Medicine, University of Florence, 50134 Florence, Italy; dR. D. Berlin Center for Cell Analysis and Modeling, University of Connecticut Health Center, Farmington, CT 06030; eDepartment of Physics and Astronomy, University of Florence, 50019 Sesto Fiorentino, Italy; and fNational Institute of Optics, National Research Council, 50125 Florence, Italy Edited by Clara Franzini-Armstrong, University of Pennsylvania Medical Center, Philadelphia, PA, and approved September 15, 2014 (received for review June 20, 2014) Action potentials (APs), via the transverse axial tubular system in a rat model of postischemic HF, structurally remodeled TATS + (TATS), synchronously trigger uniform Ca2 release throughout the exhibits abnormal electrical activity, i.e., failure of AP propagation cardiomyocyte. In heart failure (HF), TATS structural remodeling and presence of local spontaneous depolarizations. Tubular AP occurs, leading to asynchronous Ca2+ release across the myocyte failures and spontaneous activity can potentially aggravate asyn- + and contributing to contractile dysfunction. In cardiomyocytes from chronous Ca2 release and determine nonhomogeneous myofibril + failing rat hearts, we previously documented the presence of TATS contraction. Simultaneous recording of local Ca2 release and AP in elements which failed to propagate AP and displayed spontaneous the tubular network is needed to unravel the consequences of these + + electrical activity; the consequence for Ca2 release remained, how- electrical anomalies on intracellular Ca2 dynamics.