Short Notes Activity Temperatures in Oplurus Cyclurus, Oplurus Cuvieri
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Blumgart Et Al 2017- Herpetological Survey Nosy Komba
Journal of Natural History ISSN: 0022-2933 (Print) 1464-5262 (Online) Journal homepage: http://www.tandfonline.com/loi/tnah20 Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar Dan Blumgart, Julia Dolhem & Christopher J. Raxworthy To cite this article: Dan Blumgart, Julia Dolhem & Christopher J. Raxworthy (2017): Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar, Journal of Natural History, DOI: 10.1080/00222933.2017.1287312 To link to this article: http://dx.doi.org/10.1080/00222933.2017.1287312 Published online: 28 Feb 2017. Submit your article to this journal Article views: 23 View related articles View Crossmark data Full Terms & Conditions of access and use can be found at http://www.tandfonline.com/action/journalInformation?journalCode=tnah20 Download by: [BBSRC] Date: 21 March 2017, At: 02:56 JOURNAL OF NATURAL HISTORY, 2017 http://dx.doi.org/10.1080/00222933.2017.1287312 Herpetological diversity across intact and modified habitats of Nosy Komba Island, Madagascar Dan Blumgart a, Julia Dolhema and Christopher J. Raxworthyb aMadagascar Research and Conservation Institute, BP 270, Hellville, Nosy Be, Madagascar; bDivision of Vertebrate Zoology, American, Museum of Natural History, New York, NY, USA ABSTRACT ARTICLE HISTORY A six month herpetological survey was undertaken between March Received 16 August 2016 and September 2015 on Nosy Komba, an island off of the north- Accepted 17 January 2017 west coast of mainland Madagascar which has undergone con- KEYWORDS fi siderable anthropogenic modi cation. A total of 14 species were Herpetofauna; conservation; found that have not been previously recorded on Nosy Komba, Madagascar; Nosy Komba; bringing the total island diversity to 52 (41 reptiles and 11 frogs). -

Literature Cited in Lizards Natural History Database
Literature Cited in Lizards Natural History database Abdala, C. S., A. S. Quinteros, and R. E. Espinoza. 2008. Two new species of Liolaemus (Iguania: Liolaemidae) from the puna of northwestern Argentina. Herpetologica 64:458-471. Abdala, C. S., D. Baldo, R. A. Juárez, and R. E. Espinoza. 2016. The first parthenogenetic pleurodont Iguanian: a new all-female Liolaemus (Squamata: Liolaemidae) from western Argentina. Copeia 104:487-497. Abdala, C. S., J. C. Acosta, M. R. Cabrera, H. J. Villaviciencio, and J. Marinero. 2009. A new Andean Liolaemus of the L. montanus series (Squamata: Iguania: Liolaemidae) from western Argentina. South American Journal of Herpetology 4:91-102. Abdala, C. S., J. L. Acosta, J. C. Acosta, B. B. Alvarez, F. Arias, L. J. Avila, . S. M. Zalba. 2012. Categorización del estado de conservación de las lagartijas y anfisbenas de la República Argentina. Cuadernos de Herpetologia 26 (Suppl. 1):215-248. Abell, A. J. 1999. Male-female spacing patterns in the lizard, Sceloporus virgatus. Amphibia-Reptilia 20:185-194. Abts, M. L. 1987. Environment and variation in life history traits of the Chuckwalla, Sauromalus obesus. Ecological Monographs 57:215-232. Achaval, F., and A. Olmos. 2003. Anfibios y reptiles del Uruguay. Montevideo, Uruguay: Facultad de Ciencias. Achaval, F., and A. Olmos. 2007. Anfibio y reptiles del Uruguay, 3rd edn. Montevideo, Uruguay: Serie Fauna 1. Ackermann, T. 2006. Schreibers Glatkopfleguan Leiocephalus schreibersii. Munich, Germany: Natur und Tier. Ackley, J. W., P. J. Muelleman, R. E. Carter, R. W. Henderson, and R. Powell. 2009. A rapid assessment of herpetofaunal diversity in variously altered habitats on Dominica. -

MADAGASCAR: the Wonders of the “8Th Continent” a Tropical Birding Set Departure
MADAGASCAR: The Wonders of the “8th Continent” A Tropical Birding Set Departure November 3—28, 2013 Guide: Ken Behrens All photos taken during this trip. All photos by Ken Behrens unless noted otherwise. TOUR SUMMARY Madagascar has long been a core destination for Tropical Birding, and with last year’s opening of a satellite office in the country, we have further solidified our expertise in the “Eighth Continent.” This was another highly successful set-departure tour to this special island. It included both the Northwestern Endemics Pre-Trip at the start and the Helmet Vanga extension to the Masoala Peninsula at the end. Although Madagascar poses some logistical challenges, especially in the form of the national airline Air Madagascar, we had no problems on this tour, not even a single delayed flight! The birding was great, with 196 species recorded, including almost all of the island’s endemic birds. As usual, the highlight was seeing all five of the incredible ground-rollers, from the roadrunner-like Long-tailed of the spiny forest to the wonderful rainforest-dwelling Scaly. There was a strong cast of vangas, including Helmet, Bernier’s, and Sickle-billed. In fact, we saw every member of the family save the mysterious Red-tailed Newtonia which is only regularly seen in the far south. As normal, the couas were also a favorite. From the shy and beautiful Red-breasted of Madagascar Set Departure Tour Nov. 3-28, 2013 the eastern rainforest to the huge Giant Coua of the dry western forest, we were looking for and at couas virtually every day! The bizarre mesites form a Malagasy endemic family, and we had superb extended views of all three members of the family. -

The Evolution of Demographic Tactics in Lizards: a Test of Some Hypotheses Concerning Life History Evolution
J. evol. biol. 11 (1998) 329–364 1010–061X/98/030329–36 $ 1.50+0.20/0 The evolution of demographic tactics in lizards: a test of some hypotheses concerning life history evolution J. Clobert,1 T. Garland Jr.2 and R. Barbault1 1Laboratoire d’Ecologie, Uni6ersite´ Pierre et Marie Curie, Baˆtiment A, Case 237, 7 quai Saint Bernard, 75252 Paris cedex 05, France 2Department of Zoology, 430 Lincoln Dri6e, Uni6ersity of Wisconsin, Madison, WI 53706-1381, USA Key words: Comparative methods; demographic tactics; life history; phylogeny; dimension numbers; lizards. Abstract We analyze, with an augmented data base, patterns of covariation of the three primary demographic parameters (age at maturity, fecundity, adult survival, all measured in the same unit of time) in lizards. This also constitutes a first attempt to use all three of these parameters for this group of species. We attempt to place these analyses in the framework of recent theories on life history evolution (Ferrie`re and Clobert, 1992; Charnov, 1993). Life history data were collected from the literature and from our original work, and a composite phylogeny was assembled, based on a variety of published sources. Using a phylogenetically based statistical method (independent contrasts), the allometric (log-log) relationship of fecundity (and of clutch size) in relation to snout-vent length was found to differ significantly between the two major clades of extant lizards, Iguania (43 species in our data set) and Scleroglossa (47 species). We therefore emphasize analyses done separately for the two clades. Without removing correlations with body size, the relationships between fecundity and survival, and between fecundity and age at maturity, were also found to differ between clades, which differs from Charnov’s (1993) predic- tions. -

Reptiles & Amphibians of Kirindy
REPTILES & AMPHIBIANS OF KIRINDY KIRINDY FOREST is a dry deciduous forest covering about 12,000 ha and is managed by the Centre National de Formation, dʹEtudes et de Recherche en Environnement et Foresterie (CNFEREF). Dry deciduous forests are among the world’s most threatened ecosystems, and in Madagascar they have been reduced to 3 per cent of their original extent. Located in Central Menabe, Kirindy forms part of a conservation priority area and contains several locally endemic animal and plant species. Kirindy supports seven species of lemur and Madagascarʹs largest predator, the fossa. Kirindy’s plants are equally notable and include two species of baobab, as well as the Malagasy endemic hazomalany tree (Hazomalania voyroni). Ninety‐nine per cent of Madagascar’s known amphibians and 95% of Madagascar’s reptiles are endemic. Kirindy Forest has around 50 species of reptiles, including 7 species of chameleons and 11 species of snakes. This guide describes the common amphibians and reptiles that you are likely to see during your stay in Kirindy forest and gives some field notes to help towards their identification. The guide is specifically for use on TBA’s educational courses and not for commercial purposes. This guide would not have been possible without the photos and expertise of Marius Burger. Please note this guide is a work in progress. Further contributions of new photos, ids and descriptions to this guide are appreciated. This document was developed during Tropical Biology Association field courses in Kirindy. It was written by Rosie Trevelyan and designed by Brigid Barry, Bonnie Metherell and Monica Frisch. -

A Multidimensional Approach for Detecting Species Patterns in Malagasy Vertebrates
Colloquium A multidimensional approach for detecting species patterns in Malagasy vertebrates Anne D. Yoder*†, Link E. Olson‡§, Carol Hanley*, Kellie L. Heckman*, Rodin Rasoloarison¶, Amy L. Russell*, Julie Ranivo¶ʈ, Voahangy Soarimalala¶ʈ, K. Praveen Karanth*, Achille P. Raselimananaʈ, and Steven M. Goodman§¶ʈ *Department of Ecology and Evolutionary Biology, Yale University, P.O. Box 208105, New Haven, CT 06520; ‡University of Alaska Museum of the North, 907 Yukon Drive, Fairbanks, AK 99775; ¶De´partement de Biologie Animale, Universite´d’Antananarivo, BP 906, Antananarivo (101), Madagascar; ʈWWF Madagascar, BP 738, Antananarivo (101), Madagascar; and §Department of Zoology, Field Museum of Natural History, 1400 South Lake Shore Drive, Chicago, IL 60605 The biodiversity of Madagascar is extraordinarily distinctive, di- distinctiveness, especially in those cases where reproductive isola- verse, and endangered. It is therefore urgent that steps be taken tion cannot be determined for reasons of geographic separation. to document, describe, interpret, and protect this exceptional Thus, although the BSC by no means provides a universally biota. As a collaborative group of field and laboratory biologists, applicable recipe whereby the working biologist can diagnose a we employ a suite of methodological and analytical tools to species, and thus define its evolutionary significance, the concept investigate the vertebrate portion of Madagascar’s fauna. Given provides a practicable toolkit for embarking upon the enterprise. that species are the fundamental unit of evolution, where micro- As a collaborative group of field and lab biologists, we are and macroevolutionary forces converge to generate biological motivated by an interest in documenting, describing, understanding, diversity, a thorough understanding of species distribution and and preserving the endangered vertebrate biota of Madagascar. -

Zoonotic and Public Health Implications of Campylobacter Species and Squamates (Lizards, Snakes and Amphisbaenians)
pathogens Review Zoonotic and Public Health Implications of Campylobacter Species and Squamates (Lizards, Snakes and Amphisbaenians) Nicodemus M. Masila 1,2 , Kirstin E. Ross 1 , Michael G. Gardner 1,3 and Harriet Whiley 1,* 1 College of Science and Engineering, Flinders University, GPO Box 2100, Adelaide, SA 5001, Australia; [email protected] (N.M.M.); Kirstin.ross@flinders.edu.au (K.E.R.); michael.gardner@flinders.edu.au (M.G.G.) 2 Kenya Tsetse and Trypanosomiasis Eradication Council (KENTTEC), P.O. BOX 66290, Westlands, Nairobi 00800, Kenya 3 Evolutionary Biology Unit, South Australian Museum, North Terrace, Adelaide, SA 5000, Australia * Correspondence: harriet.whiley@flinders.edu.au; Tel.: +61-87-2218-580 Received: 26 August 2020; Accepted: 25 September 2020; Published: 28 September 2020 Abstract: Campylobacter spp. is one of the most widespread infectious diseases of veterinary and public health significance. Globally, the incidence of campylobacteriosis has increased over the last decade in both developing and developed countries. Squamates (lizards, snakes and amphisbaenians) are a potential reservoir and source of transmission of campylobacteriosis to humans. This systematic review examined studies from the last 20 years that have reported squamate-associated human campylobacteriosis. It was found that C. fetus subsp. testudinum and C. fetus subsp. fetus were the most common species responsible for human campylobacteriosis from a squamate host. The common squamate hosts identified included bearded dragons (Pogona vitticeps), green iguana (Iguana iguana), western beaked gecko (Rhynchoedura ornate) and blotched blue-tongued skink (Tiliqua nigrolutea). People with underlying chronic illnesses, the immunocompromised and the elderly were identified as the most vulnerable population. -

The Helminth Fauna of Apathya Cappadocica (Werner, 1902) (Anatolian Lizard) (Squamata: Lacertidae) from Turkey
©2015 Institute of Parasitology, SAS, Košice DOI 10.1515/helmin-2015-0049 HELMINTHOLOGIA, 52, 4: 310 – 315, 2015 The helminth fauna of Apathya cappadocica (Werner, 1902) (Anatolian Lizard) (Squamata: Lacertidae) from Turkey S. BIRLIK1, H. S. YILDIRIMHAN1, N. SÜMER1, Ç. ILGAZ2, Y. KUMLUTAŞ2, Ö. GÜÇLÜ3, S. H. DURMUŞ4 1Uludag University, Faculty of Arts and Sciences, Department of Biology, Nilüfer, Bursa, Turkey; 2Dokuz Eylül University, Faculty of Science, Department of Biology, 35160, Buca-İzmir, Turkey, E-mail: [email protected]; 3Aksaray University, Güzelyurt Vocational School, Department of Plant and Animal Production, 68500, Güzelyurt/Aksaray, Turkey; 4Dokuz Eylül University, Faculty of Education, Department of Biology, 35160, Buca-İzmir, Turkey Article info Summary Received May 28, 2015 A total of thirty-one Anatolian Lizard, Apathya cappacocica, samples from several provinces of East- Accepted June 4, 2015 ern and South-Eastern Turkey were examined for helminths. Two species of Nematoda, including Spauligodon atlanticus, Skrjabinodon medinae; two species of Cestoda, including Mesocestoides sp. tetrahydia and Oochoristica tuberculata and one species of Acanthocephala, Centrorhynchus sp. were found. This is the fi rst helminth record of A. cappodocica from Turkey. A. cappadocica represents a new host record for each of the parasite species. S. atlanticus is reported from Turkey for the fi rst time. Keywords: Nematoda; Cestoda; Acanthocephala; Anatolian lizard; Apathya cappadocica; Turkey Introduction vilacerta parva (Saygı & Olgun, 1993); Crimean Wall Lizard, Po- darcis tauricus (Schad et al., 1960); Pleske’s Racerunner-Trans- The Anatolian Lizard, Apathya cappadocica (Werner 1902) is caucasian Racerunner, Eremias pleskei, Strauch’s Racerunner, found in Turkey (central, eastern, southern and southeastern Ana- Eremias strauchi, Suphan Racerunner, Eremias suphani (Düsen tolia), northern Syria, northern Iraq, and northwestern Iran (Ilgaz et al., 2013); Ocellated Skink Chalcides ocellatus (Incedogan et et al., 2010; Baran et al., 2012). -

Generic Relationships Within Cordyliformes (Reptilia . Squamata)
I I BULLETIN DE L'INSTITUT ROYAL DES SCIENCES NATURELLES DE BELGIQUE, BIOLOGIE, 61:121-188, 199 1 BULLETIN VAN HET KONINKL!JK BELGISCH INSTITUUT YOOR NATUURWETENSCHAPPEN, BIOLOGIE, 61:121-1 88, 1991 Generic relationships within Cordyliformes (Reptilia . Squamata) by Mathias LANG Table of Contents comorpha as the sister-taxon of Scincidae is accepted. A phylogenetic hypothesis of generic relationships is proposed based on 74 character complexes. Within Gerrhosauridae, the Madagascan clade Trachelop Abstract . 121 tychus-Zonosaurus represents a single speciation event coinciding with Zusammenfassung . 12 1 the separation of Madagascar from Africa. Within African gerrhosau Introduction : Historical Review of the taxonomy and proposed rids, the monotypic Angolosaurus is regarded as the earliest diverging affinities of Cordylidae + Gerrhosauridae 122 taxon. Gerrhosaurus is the sister-taxon to a Cordylosaurus-Tetradac Monophyly of Cordylidae + Gerrhosauridae 123 tylus clade. Within the purely African Cordylidae, the serpentine Cha Goals and problems of this study 124 maesaura is the earliest diverging taxon. Cordylus is the sister-taxon Material and Methods . 124 to a Platysaurus-Pseudocordylus clade. A new classification is propo Specimens . 124 sed based on these phylogenetic patterns. This classification parallels Outgroup comparison 124 taxonomic categories within the Scincomorpha. Biogeographical pat Systematic Characters 125 terns are evaluated in light of the proposed phylogeny and compared Scale characters 126 with Chamaeleonidae, -

Captive Wildlife Regulations, 2021, W-13.12 Reg 5
1 CAPTIVE WILDLIFE, 2021 W-13.12 REG 5 The Captive Wildlife Regulations, 2021 being Chapter W-13.12 Reg 5 (effective June 1, 2021). NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 W-13.12 REG 5 CAPTIVE WILDLIFE, 2021 Table of Contents PART 1 PART 5 Preliminary Matters Zoo Licences and Travelling Zoo Licences 1 Title 38 Definition for Part 2 Definitions and interpretation 39 CAZA standards 3 Application 40 Requirements – zoo licence or travelling zoo licence PART 2 41 Breeding and release Designations, Prohibitions and Licences PART 6 4 Captive wildlife – designations Wildlife Rehabilitation Licences 5 Prohibition – holding unlisted species in captivity 42 Definitions for Part 6 Prohibition – holding restricted species in captivity 43 Standards for wildlife rehabilitation 7 Captive wildlife licences 44 No property acquired in wildlife held for 8 Licence not required rehabilitation 9 Application for captive wildlife licence 45 Requirements – wildlife rehabilitation licence 10 Renewal 46 Restrictions – wildlife not to be rehabilitated 11 Issuance or renewal of licence on terms and conditions 47 Wildlife rehabilitation practices 12 Licence or renewal term PART 7 Scientific Research Licences 13 Amendment, suspension, -
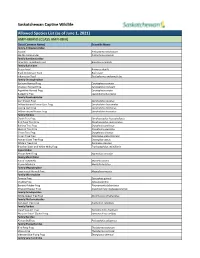
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Madagascar Highlights Birding and Wildlife Tour Trip Description and Itinerary
Madagascar Highlights Birding and Wildlife Tour Trip Description and Itinerary 15 Days and 14 Nights November 2 to 16, 2020 Brief Itinerary November 2: Arrival and Birding Lac Alarobia Overnight at San Cristobal Hotel in Tana. November 3: Fly to Tulear and Drive to Ifaty; and Late PM Start Birding in Thorn Forest and/or nearby Areas Overnight at Bamboo Hotel in Ifaty. November 4: Birding Full Day in Ifaty: AM Didierea Woodland and PM Wetlands Birding Overnight at Bamboo Hotel in Ifaty. November 5: Ifaty to Tulear: Key Birding Trip by Boat to Nosy Ve and San Augustin, and Birding to La Table Mountain Overnight at Auberge de la Table in Tulear. November 6: AM Very Early Start for Full Day Birding: Zombitse Forest and then Isalo National Park Overnight at Jardin du Roy in Isalo. November 7: Bit of Birding on Long Drive to Ranomafana National Park November 8 and 9: Two Full Days Birding (and wildlife watching) at Ranomafana National Park November 10: Another Bonus Full Day Birding (and wildlife watching) at Ranomafana National Park November 11: Long Day in Vehicle Driving from Ranomafana to Antananarivo November 12: AM Drive to Perinet (with some birding along the way); PM Birding Perinet Overnight at Sahatandra River Hotel in Perinet. November 13 and 14: Perinet Special Reserve and Mantadia National Park Both nights, we overnight at Sahatandra River Hotel in Perinet. November 15: AM Final Perinet Birding and Wildlife Watching or Try for Helmet Vanga; PM Back to Tana Overnight at San Cristobal Hotel in Tana. November 16: Departures from Tana DETAILED ITINERARY: November 2: The Group Arrives and PM Birding Start at Lac Alarobia Most of the group will come in this morning before 1:00 PM (and we can deal with flights as late as 2 PM today).