Ajbd1200554 1234..1254
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pbv1 105.Pdf
CONIFER DOMINANTS IN THE MIDDLE TERTIARY OF THE JOHN DAY BASIN, OREGON RALPH w. CHANEY Professor of Palaeontology, University of California, and Research Associate, Carnegie Institution o[ Washington FOREWORD _ During the past few years, most of my time has been devoted to a re-study of all URING the early weeks of 1948, the available material of Sequoia and it was my good fortune to be in Taxodium from the Cretaceous and Tertiary D daily contact with Birbal Sahni who of North America. Many of the fossil was then a visitor at the University of specimens previously assigned to these California. We had many discussions of genera are now known to represen t M eta Metasequoia, the conifer whose discovery sequoia. The characters by which the in Asia a few years before was proving of leaves and cones of this genus, both living such great interest to botanists and palaeo and fossil, may be recognized have been botanists. My departure for central China considered by Miki, Hu and Cheng, Stebbins in February to visit scattered groves of ( 1948, p. 96), and Chaney ( 1948, p. 509; this survivor from the past brought to a 1951, pp. 174-181, PL. 6). It will b~ suffi close an enjoyable and profitable opportunity cient at this point to note that a decussate to consider problems of Indian and North arrangement is characteristic of Metasequoia, American palaeobotany with a leading stu by which foliage shoots and needles and dent of science in Asia. I shall always be cones, both pistillate and staminate, may be happy to recall that on the night of my readily distinguished from those of Sequoia departure from San Francisco, Dr. -

John Day Fossil Beds NM: Geology and Paleoenvironments of the Clarno Unit
John Day Fossil Beds NM: Geology and Paleoenvironments of the Clarno Unit JOHN DAY FOSSIL BEDS Geology and Paleoenvironments of the Clarno Unit John Day Fossil Beds National Monument, Oregon GEOLOGY AND PALEOENVIRONMENTS OF THE CLARNO UNIT John Day Fossil Beds National Monument, Oregon By Erick A. Bestland, PhD Erick Bestland and Associates, 1010 Monroe St., Eugene, OR 97402 Gregory J. Retallack, PhD Department of Geological Sciences University of Oregon Eugene, OR 7403-1272 June 28, 1994 Final Report NPS Contract CX-9000-1-10009 TABLE OF CONTENTS joda/bestland-retallack1/index.htm Last Updated: 21-Aug-2007 http://www.nps.gov/history/history/online_books/joda/bestland-retallack1/index.htm[4/18/2014 12:20:25 PM] John Day Fossil Beds NM: Geology and Paleoenvironments of the Clarno Unit (Table of Contents) JOHN DAY FOSSIL BEDS Geology and Paleoenvironments of the Clarno Unit John Day Fossil Beds National Monument, Oregon TABLE OF CONTENTS COVER ABSTRACT ACKNOWLEDGEMENTS CHAPTER I: INTRODUCTION AND REGIONAL GEOLOGY INTRODUCTION PREVIOUS WORK AND REGIONAL GEOLOGY Basement rocks Clarno Formation John Day Formation CHAPTER II: GEOLOGIC FRAMEWORK INTRODUCTION Stratigraphic nomenclature Radiometric age determinations CLARNO FORMATION LITHOSTRATIGRAPHIC UNITS Lower Clarno Formation units Main section JOHN DAY FORMATION LITHOSTRATIGRAPHIC UNITS Lower Big Basin Member Middle and upper Big Basin Member Turtle Cove Member GEOCHEMISTRY OF LAVA FLOW AND TUFF UNITS Basaltic lava flows Geochemistry of andesitic units Geochemistry of tuffs STRUCTURE OF CLARNO -

Geological Survey of Austria ©Geol
©Geol. Bundesanstalt, Wien; download unter www.geologie.ac.at und www.zobodat.at Berichte der Geologischen Bundesanstalt, 120 Berichte der Geologischen Bundesanstalt, Benjamin Sames (Ed.) th 10 International Symposium on the Cretaceous: ABSTRACTS Berichte der Geologischen Bundesanstalt, 120 www.geologie.ac.at Geological Survey of Austria ©Geol. Bundesanstalt, Wien; download unter www.geologie.ac.at und www.zobodat.at Berichte der Geologischen Bundesanstalt (ISSN 1017-8880) Band 120 10th International Symposium on the Cretaceous Vienna, August 21–26, 2017 — ABSTRACTS BENJAMIN SAMES (Ed.) ©Geol. Bundesanstalt, Wien; download unter www.geologie.ac.at und www.zobodat.at Berichte der Geologischen Bundesanstalt, 120 ISSN 1017-8880 Wien, im Juli 2017 10th International Symposium on the Cretaceous Vienna, August 21–26, 2017 – ABSTRACTS Benjamin Sames, Editor Dr. Benjamin Sames, Universität Wien, Department for Geodynamics and Sedimentology, Center for Earth Sciences, Althanstraße 14, 1090 Vienna, Austria. Recommended citation / Zitiervorschlag Volume / Gesamtwerk Sames, B. (Ed.) (2017): 10th International Symposium on the Cretaceous – Abstracts, 21–26 August 2017, Vienna. – Berichte der Geologischen Bundesanstalt, 120, 351 pp., Vienna. Abstract (example / Beispiel) Granier, B., Gèze, R., Azar, D. & Maksoud, S. (2017): Regional stages: What is the use of them – A case study in Lebanon. – In: Sames, B. (Ed.): 10th International Symposium on the Cretaceous – Abstracts, 21–26 August 2017, Vienna. – Berichte der Geologischen Bundesanstalt, 120, 102, Vienna. Cover design: Monika Brüggemann-Ledolter (Geologische Bundesanstalt). Cover picture: Postalm section, upper Campanian red pelagic limestone-marl cycles (CORBs) of the Nierental Formation, Gosau Group, Northern Calcareous Alps (Photograph: M. Wagreich). 10th ISC Logo: Benjamin Sames The 10th ISC Logo is composed of selected elements of the Viennese skyline with, from left to right, the Stephansdom (St. -

Taphonomic and Depositional Analysis of Megaflora of the Cretaceous Hell Creek Formation Near Marmarth, North Dakota
OpenRiver Student Research and Creative Projects 2016-2017 Grants & Sponsored Projects 9-1-2016 Taphonomic and Depositional Analysis of Megaflora of the Cretaceous Hell Creek Formation near Marmarth, North Dakota Nelson Mallory Winona State University Chelsea Ames Winona State University Will Franta Winona State University Follow this and additional works at: https://openriver.winona.edu/studentgrants2017 Recommended Citation Mallory, Nelson; Ames, Chelsea; and Franta, Will, "Taphonomic and Depositional Analysis of Megaflora of the Cretaceous Hell Creek Formation near Marmarth, North Dakota" (2016). Student Research and Creative Projects 2016-2017. 3. https://openriver.winona.edu/studentgrants2017/3 This Grant is brought to you for free and open access by the Grants & Sponsored Projects at OpenRiver. It has been accepted for inclusion in Student Research and Creative Projects 2016-2017 by an authorized administrator of OpenRiver. For more information, please contact [email protected]. Taphonomic and Depositional Analysis of Megaflora of the Cretaceous Hell Creek Formation near Marmarth, North Dakota Mallory Nelson, Chelsea Ames, Will Franta Department of Geoscience, Winona State University, Winona, Minnesota 55987 ABSTRACT The Hell Creek formation of western North Dakota and eastern Montana contains a large variety of plant, pollen, and vertebrate fossils. A high quality plant fossil site near Marmarth, North Dakota was studied to obtain megafloral fossils characteristic of the unit. Plant fossils from the unit were obtained, photographed, and identified so as to obtain a more thorough knowledge of the depositional environment and climate of the region during the Cretaceous period. Fossils identified indicate a forested, deltaic environment rich in water and sediment influx. Introduction been extensively sought after and analyzed by The Hell Creek formation of Western North paleontologists, plant and pollen fossils receive far Dakota and Eastern Montana has long been less study (Johnson, 330). -

Palaeogene Deposits and the Platform Structure of Svalbard
NORSK POLARINSTITUTT SKRIFTER NR. 159 v JU. JA. LIVSIC Palaeogene deposits and the platform structure of Svalbard NORSK POLARINSTITUTT OSLO 1974 DET KONGEUGE DEPARTEMENT FOR INDUSTRI OG IlANDVERK NORSK POLARINSTITUTT Rolfstangveien 12, Snareya, 1330 Oslo Lufthavn, Norway SALG AV B0KER SALE OF BOOKS Bekene selges gjennom bokhandlere, eller The books are sold through bookshops, or bestilles direkte fra : may be ordered directly from: UNlVERSITETSFORLAGET Postboks 307 16 Pall Mall P.O.Box 142 Blindem, Oslo 3 London SW 1 Boston, Mass. 02113 Norway England USA Publikasjonsliste, som ogsa omfatter land List of publications, including maps and og sjekart, kan sendes pa anmodning. charts, wiZ be sent on request. NORSK POLARINSTITUTT SKRIFTER NR.159 JU. JA. LIVSIC Palaeogene deposits and the platform structure of Svalbard NORSK POLARINSTITUTT OSLO 1974 Manuscript received March 1971 Published April 1974 Contents Abstract ........................ 5 The eastern marginal fault zone 25 The Sassendalen monocline ...... 25 AHHOTaU;HH (Russian abstract) 5 The east Svalbard horst-like uplift 25 The Olgastretet trough .. .. .. .. 26 Introduction 7 The Kong Karls Land uplift .... 26 Stratigraphy ..................... 10 The main stages of formation of the A. Interpretation of sections ac- platform structure of the archipelago 26 cording to areas ............ 11 The Central Basin ........... 11 The Forlandsundet area ..... 13 The importance of Palaeogene depo- The Kongsfjorden area ....... 14 sits for oil and gas prospecting in The Renardodden area ...... 14 Svalbard .. .. .. .. .. .. .. 30 B. Correlation of sections ....... 15 Bituminosity and reservoir rock C. Svalbard Palaeogene deposits properties in Palaeogene deposits 30 as part of the Palaeogene depo- Hydrogeological criteria testifying sits of the Polar Basin ...... 15 to gas and oil content of the rocks 34 Tectonic criteria of oil and gas con- Mineral composition and conditions of tent . -

A Review of Paleobotanical Studies of the Early Eocene Okanagan (Okanogan) Highlands Floras of British Columbia, Canada and Washington, USA
Canadian Journal of Earth Sciences A review of paleobotanical studies of the Early Eocene Okanagan (Okanogan) Highlands floras of British Columbia, Canada and Washington, USA. Journal: Canadian Journal of Earth Sciences Manuscript ID cjes-2015-0177.R1 Manuscript Type: Review Date Submitted by the Author: 02-Feb-2016 Complete List of Authors: Greenwood, David R.; Brandon University, Dept. of Biology Pigg, KathleenDraft B.; School of Life Sciences, Basinger, James F.; Dept of Geological Sciences DeVore, Melanie L.; Dept of Biological and Environmental Science, Keyword: Eocene, paleobotany, Okanagan Highlands, history, palynology https://mc06.manuscriptcentral.com/cjes-pubs Page 1 of 70 Canadian Journal of Earth Sciences 1 A review of paleobotanical studies of the Early Eocene Okanagan (Okanogan) 2 Highlands floras of British Columbia, Canada and Washington, USA. 3 4 David R. Greenwood, Kathleen B. Pigg, James F. Basinger, and Melanie L. DeVore 5 6 7 8 9 10 11 Draft 12 David R. Greenwood , Department of Biology, Brandon University, J.R. Brodie Science 13 Centre, 270-18th Street, Brandon, MB R7A 6A9, Canada; 14 Kathleen B. Pigg , School of Life Sciences, Arizona State University, PO Box 874501, 15 Tempe, AZ 85287-4501, USA [email protected]; 16 James F. Basinger , Department of Geological Sciences, University of Saskatchewan, 17 Saskatoon, SK S7N 5E2, Canada; 18 Melanie L. DeVore , Department of Biological & Environmental Sciences, Georgia 19 College & State University, 135 Herty Hall, Milledgeville, GA 31061 USA 20 21 22 23 Corresponding author: David R. Greenwood (email: [email protected]) 1 https://mc06.manuscriptcentral.com/cjes-pubs Canadian Journal of Earth Sciences Page 2 of 70 24 A review of paleobotanical studies of the Early Eocene Okanagan (Okanogan) 25 Highlands floras of British Columbia, Canada and Washington, USA. -

1145 Akhmetiev.Vp
Paleocene and Eocene floristic and climatic change in Russia and Northern Kazakhstan MIKHAIL A. AKHMETIEV Paleocene and Eocene floras of Russia and adjacent regions are reviewed with an interpretation of climatic conditions under which they developed. Floristic and climatic changes in western and central regions of Russia in the Paleocene and in the first part of the Eocene were caused by the dynamics and rearrangement of the systems of marine seaways: a longi- tudinal seaway, which connected the Arctic basin with the marginal seas of Northern Peri-Tethys (Turanian, South Rus- sian and others), and a latitudinal seaway, which connected the marginal seas of Northern Peri-Tethys with the Atlantic Ocean. As these systems were progressively reduced, the climate in the middle latitudes changed from paratropical (like in West and Central Europe) to a subtropical monsoon climate with wet summers (Late Ypresian to Lutetian), and later to a climate with wet winters (Late Lutetian to the first part of the Priabonian). Floristic changes reflect these climatic trends. In the Paleogene, cold currents constantly influenced the climate of regions of the northwestern Pacific and facili- tated development of a warm-temperate mesophilic flora. A warmer episode took place in the Early Eocene. At this time thermophilic plants (Sabal, Myrtaceae and Lauraceae) reached Koryakia, North Western Kamchatka, probably as a re- sult of northward migration. Some subtropical plants existed near the Recent Polar circle. The subtropical Raichikha-type Flora lost temperate elements and, by ecological and climatic types, it is more similar to the Recent flora of South-West and South China. -

Ore Bin / Oregon Geology Magazine / Journal
State of Oregon The ORE BIN Department of Geology Volume 35, No.3 and Mineral Industries 1069State Office Bldg. March 1973 Portland Oregon 97201 THE OLIGOCENE LYONS FLORA OF NORTHWESTERN OREGON Herb Meyer Student, Portland State University The purpose of this study of the Lyons flora is to determine the age and paleoecology of the flora through the examination and identification of the fossil plant species of the flora. The plant fossi Is comprising the Lyons flora were collected from a locality in the .t~~·fi:~ora upper Thomas Creek area, 5 mi les sou theast of the town of Lyons, Oregon. Geologic Occurrence The beds from which the Lyons flora was obtained are part of the Little Butte Volcanic Series of Oligocene and early Miocene age described by Peck and others(1964). Stratigraphically below the fossil deposit, the Little Butte Volcanic Series is characterized by a pumiceous tuff-breccia which contains blocks and fragments of a volcanic flow rock. This exposure, the base of which is not exposed, underlies the fossil deposit for a thickness of more than 400 feet. The deposit containing the fossi I leaves is composed of a thinly lami nated tuffaceous material which has been silicifed to varying degrees. These beds may have been deposi ted in a sha !low, quiet body of water. Lacustrine deposition is suggested by the stratification of the beds, the abundant pres ence of fossil leaves, and the presence of one water plant in the fossil record. Composition of the Lyons Flora Twenty-four identified fossi I plants represent the Lyons flora as it is known at this point in the study. -

OREGON GEOLOGY Published by The
OREGON GEOLOGY published by the Oregon Department of Geology and Mineral Industries 1931 VOLUME 58, NUMBER 3 MAY 1996 Hyrachus eximius Alnus heterodonta Telmatherium manteoceras Orohippus major corsonr Patriofelis ferox IN THIS ISSUE: Reconstructions of Eocene and Oligocene Plants and Animals of Central Oregon OREGON GEOLOGY In memoriam: Volunteer Shirley O'Dell IISSN 0164-3304) Shirley O'Dell, volunteer at the Nature of the North west Information Center, died March 30, 1996. VOLUME 58, NUMBER 3 MAY 1996 Born in Minneapolis, Minnesota, she spent most of her Published bimonthly in Jaooary, Mardl, May, July, Sq:Kember, Md November by the Oregon Dcp.vncn of life in Portland, Oregon. She was a secretary at Charles F. Geology and MintnI IrnkJstries. (Volumes 1 tl'I'ough 40 wereemtJed The On Bin.) Berg, at an investment company, and at Columbia Grain Governing Board John W. Stephens, Chair .......................................................... Portland Company. After her retirement, she worked as a volunteer Jacqueline G. Haggerty ............................................ Weston Mountain at St. Vincent's Medical Center Gift Shop and at the Na Ronald K. Culbertson ...................................................... Myrtle Creek ture of the Northwest Infomation Center. She was a mem ber of the Geological Society of the Oregon Country State Geologist ............................................................ Donald A Hull Deputy State Geologist ........................................... John D. Beaulieu (GSOC) for more than 30 years -

54-59 6 S. Knoll Schwanz-Mammutbaum.Pdf
Helmut Knoll & Harald Schwanz Mammutbäume – lebende Fossilien Als lebende Fossilien bezeichnet man Lebewe- sen, die schon seit Jahrmillionen auf der Erde leben und sich in diesem langen Zeitraum kaum verändert haben, wie den Quastenfl osser. Es gibt aber auch im Pfl anzenreich lebende Fossilien, die Mammutbäume. In manchen Fällen waren sie schon aus fossilen Funden bekannt, ehe man die ersten lebenden Exemplare wieder entdeckte. Mammutbäume in herrlichen Parkanlagen Viele historische Parkanlagen mit exotischen Bäu- men haben wir dem Adel zu verdanken. Kaiser, Könige und was Rang und Namen hatte, traf sich dort zur Sommerfrische. Sie ließen Schlösser und Villen mit herrlichen Parkanlagen bauen. Man- che dieser Gebäude bestehen heute noch und sind genutzt, andere leider dem Verfall preisgegeben. Die Riesenmammutbäume (Sequoiadendron gigan- teum, 1, 2), gelegentlich auch Berg-Mammutbaum oder Wellingtonie genannt, blieben bestehen, und manche davon sind schon über 150 Jahre alt. Diese findet man relativ leicht. Man muss nur den Kopf und die Augen heben und oberhalb der üblichen Baumhöhen suchen, dann fallen sie auf, denn sie sind majestätisch. Wenn man sie lässt, werden sie zu wahren Giganten der Natur. Auch ist das Ausse- hen ganz speziell, wer darauf achtet, wird die Bäume ebenfalls sofort erkennen. Die Standorte der meis- ten alten Mammutbäume sind europaweit gleich- artig. Immer finden sie sich in Verbindung mit Klös- tern, Schlössern oder Villen, die ab der Mitte des 19. Jahrhunderts erbaut oder renoviert wurden. 1: Ein Riesenmammutbaum Damals herrschte der Modetrend, einen Englischen Sequoiadendron giganteum Park oder einen Schlosspark anzulegen. In diesen (Lindley) Buchholz 1939, im Landschaftsgärten sind, mit gewissen Variationen, Schlosspark Badenweiler, immer wieder dieselben Arten an exotischen Bäu- Baden-Württemberg. -

Cupressaceae) Fossils from the Eocene Buchanan Lake Formation, Axel Heiberg Island, Nunavut, Canada Reflect Diagenesis from Terpenoids of Their Related Extant Species
Review of Palaeobotany and Palynology 235 (2016) 81–93 Contents lists available at ScienceDirect Review of Palaeobotany and Palynology journal homepage: www.elsevier.com/locate/revpalbo Biomarker compositions of Glyptostrobus and Metasequoia (Cupressaceae) fossils from the Eocene Buchanan Lake Formation, Axel Heiberg Island, Nunavut, Canada reflect diagenesis from terpenoids of their related extant species Bernd R.T. Simoneit a,⁎,AngelikaOttob,NorihisaKusumotoc,JamesF.Basingerd a Department of Chemistry, Oregon State University, Corvallis, OR 97331, USA b Forschungsinstitut Senckenberg, Sektion Paläobotanik, Senckenberganlage 25, 60325 Frankfurt/Main, Germany c Forestry and Forest Products Research Institute, 1 Matsunosato, Tsukuba, Ibaraki 305-8687, Japan d Department of Geological Sciences, University of Saskatchewan, 114 Science Place, Saskatoon, Saskatchewan, S7N 5E2, Canada article info abstract Article history: The solvent extractable organic compounds of two fossil conifer species from the Eocene Buchanan Lake Forma- Received 4 December 2015 tion, Axel Heiberg Island, Canada, were compared to the compound composition of related extant species to in- Received in revised form 7 July 2016 vestigate the preservation and degradation (diagenesis) of terpenoid biomarkers in geological samples. Mono-, Accepted 19 July 2016 di- and triterpenoids, lignin components and aliphatic lipids were identified in the solvent extracts of the fossil Available online 1 September 2016 and extant conifers. The biomarker composition of fossil Glyptostrobus shoots and seed cones is different from their pattern observed in fossil Metasequoia. The comparison of terpenoids found in fossil plant extracts with Keywords: fi Biomarkers those present in their related extant species shows similar patterns of terpenoid biomarkers and con rms the Buchanan Lake Formation systematic assignment of fossil species based on morphological and anatomic characteristics. -
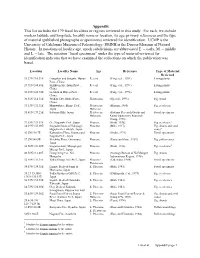
Appendix This List Includes the 179 Fossil Localities Or Regions Reviewed in This Study
Appendix This list includes the 179 fossil localities or regions reviewed in this study. For each, we include modern latitude and longitude, locality name or location, its age, primary references and the type of material (published photographs or specimens) reviewed for identification. UCMP is the University of California Museum of Paleontology; DMNH is the Denver Museum of Natural History. In notations of locality age, epoch subdivisions are abbreviated E. = early, M. = middle and L. = late. The notation “fossil specimens” under the type of material reviewed for identification indicates that we have examined the collections on which the publication was based. Location Locality Name Age Reference Type of Material Reviewed 29.37N/110.31E Longshan and Sangzhi, Hunan Recent (Ying et al., 1994) Living plants Prov., China 29.91N/108.65E Shizhu in Szechuan Prov., Recent (Ying et al., 1994) Living plants China 30.25N/108.94E Lichuan in Hupen Prov., Recent (Ying et al., 1994) Living plants China 30.51N/114.31E Wuhan City, Hubei Prov., Pleistocene (Qi et al., 1993) Fig. wood China 33.13N/132.32E Mizuwakare, Ehime Pref., Pleistocene- (Mizuno, 1980) Fig. seed cone Japan Holocene 35.41N/139.25E Sayama Hills, Japan Pleistocene- (Saitama Research Group and Fossil specimens Holocene Kanto Quaternary Research Group, 1970) 32.45N/129.52E Oe, Nagasaki Pref., Japan Pliocene (Endo, 1936) Fig. seed cone1 34.39N/135.00E Stegodon beds at Nakayagi- Pliocene (Miki, 1937) Fig. shoots and seed Higashiei bei Akashi, Japan. cones2 36.2N/138.7E Kabutoiwa Flora, Gunma and Pliocene (Ozaki, 1991) Fossil specimens Nagano Pref., Japan 37.2N/140.8E Shichiku Flora, Ono-mura, Pliocene (Tanai and Onoe, 1959) Fig.