The Mechanical Properties of Glass Theoretical Strength, Practical Strength, Fatigue, Flaws, Toughness, Chemical Processes
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tensile Strength of C/C Composites
16 TH INTERNATIONAL CONFERENCE ON COMPOSITE MATERIALS TENSILE STRENGTH OF C/C COMPOSITES Jun Koyanagi*, Hiroshi Hatta*, Yasuo Kogo**, Ichiro Shiota*** *Institute of Space and Astronautical Science, Japan Aerospace Exploration Agency, **Department of Materials Science and Technology, Tokyo University of Science *** Department of Materials Science and Technology, Kogakuin University Keywords : C/C composites, tensile strength, fracture mechanism ABSTRACT changing heat treatment temperature, and the tensile Fracture mechanism and model based on it in strength and interfacial strength were measured. In Carbon-carbon composites are studied. Interfacial addition, comparing these results with their fracture strength between reinforcing fiber and matrix is a patterns, a tensile fracture model of C/Cs was potential candidate governing the tensile fracture of proposed. C/Cs. However, The mechanisms connecting the tensile strength and the factor have not yet been 2. EXPERIMENTAL PROCEDURE clarified. In the present study, tensile fracture 2.1 Materials patterns of various C/C composites with changing Two types of C/Cs were examined. These the interfacial strength were observed in detail, and C/Cs were different only in their reinforcing fibers, a fundamental tensile fracture model of C/C K321 and K633 by Mitsubishi Sanshi Co. Japan. composites in accordance with the experimental These fibers were fabricated from the same observations were proposed. The analytical result precursor but differed only in HTT. Hereafter, the shows reasonable agreement with the experimental C/Cs reinforced with K321 and K633 will be results, and verifies that the fracture mechanism is referred to as K321-C/C and K633-C/C, respectively. consistent. Both C/Cs were of a symmetric 0°/90° cross-ply lamination, fabricated from carbon fiber reinforced phenolic resin matrix composites, CFRPs, with a 1. -

A Study of the Mechanical Properties of Composite Materials with a Dammar-Based Hybrid Matrix and Two Types of Flax Fabric Reinforcement
polymers Article A Study of the Mechanical Properties of Composite Materials with a Dammar-Based Hybrid Matrix and Two Types of Flax Fabric Reinforcement Dumitru Bolcu † and Marius Marinel St˘anescu*,† Department of Mechanics, University of Craiova, 165 Calea Bucure¸sti,200620 Craiova, Romania; [email protected] * Correspondence: [email protected]; Tel.: +40-740-355-079 † These authors contributed equally to this work. Received: 7 July 2020; Accepted: 22 July 2020; Published: 24 July 2020 Abstract: The need to protect the environment has generated, in the past decade, a competition at the producers’ level to use, as much as possible, natural materials, which are biodegradable and compostable. This trend and the composite materials have undergone a spectacular development of the natural components. Starting from these tendencies we have made and studied from the point of view of mechanical and chemical properties composite materials with three types of hybrid matrix based on the Dammar natural hybrid resin and two types of reinforcers made of flax fabric. We have researched the mechanical properties of these composite materials based on their tensile strength and vibration behavior, respectively. We have determined the characteristic curves, elasticity modulus, tensile strength, elongation at break, specific frequency and damping factor. Using SEM (Scanning Electron Microscopy) analysis we have obtained images of the breaking area for each sample that underwent a tensile test and, by applying FTIR (Fourier Transform Infrared Spectroscopy) and EDS (Energy Dispersive Spectroscopy) analyzes, we have determined the spectrum bands and the chemical composition diagram of the samples taken from the hybrid resins used as a matrix for the composite materials under study. -

Space Elevator a New Way to Gain Orbit Adil Oubou 5/3/2014
Space Elevator A New Way To Gain Orbit Adil Oubou 5/3/2014 Abstract After 56 years of great space exploration, the space flight program found itself asking an intriguing question: What’s next? Should humans constrain the space program to low Earth orbit, revisit the moon, or simply go beyond anything they have done before? No matter what the next step is, there is a great need for a reliable, safe, affordable and easy method of gaining orbit. The space elevator concept discussed in this report, based on the design provided in Peter Swan’s book “Space Elevators: An Assessment of the Technological Feasibility and the Way Forward“, shines the light on an economically and technologically feasible solution within the next two decades that will lift payloads including humans from the earth’s surface into space at a greater capacity than current rockets while significantly lowering cost to $500/kg. The system design includes a tether line that extends from the Earth’s surface to an altitude of 100,000 km that utilizes electrical motor driven climbers to gain orbit. The success feasibility of this space flight system is highly dependent on the development of Carbon Nanotube (CNT) material, which will provide high strength and low mass properties for the space elevator system components to withstand environmental and operational stresses. Table of Contents 1.0 The New Way to Orbit ....................................................................................................... 4 2.0 Why Space Elevator? ........................................................................................................ -
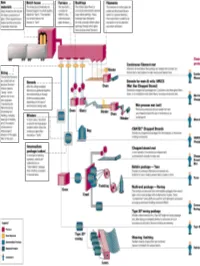
All About Fibers
RawRaw MaterialsMaterials ¾ More than half the mix is silica sand, the basic building block of any glass. ¾ Other ingredients are borates and trace amounts of specialty chemicals. Return © 2003, P. Joyce BatchBatch HouseHouse && FurnaceFurnace ¾ The materials are blended together in a bulk quantity, called the "batch." ¾ The blended mix is then fed into the furnace or "tank." ¾ The temperature is so high that the sand and other ingredients dissolve into molten glass. Return © 2003, P. Joyce BushingsBushings ¾The molten glass flows to numerous high heat-resistant platinum trays which have thousands of small, precisely drilled tubular openings, called "bushings." Return © 2003, P. Joyce FilamentsFilaments ¾This thin stream of molten glass is pulled and attenuated (drawn down) to a precise diameter, then quenched or cooled by air and water to fix this diameter and create a filament. Return © 2003, P. Joyce SizingSizing ¾The hair-like filaments are coated with an aqueous chemical mixture called a "sizing," which serves two main purposes: 1) protecting the filaments from each other during processing and handling, and 2) ensuring good adhesion of the glass fiber to the resin. Return © 2003, P. Joyce WindersWinders ¾ In most cases, the strand is wound onto high-speed winders which collect the continuous fiber glass into balls or "doffs.“ Single end roving ¾ Most of these packages are shipped directly to customers for such processes as pultrusion and filament winding. ¾ Doffs are heated in an oven to dry the chemical sizing. Return © 2003, P. Joyce IntermediateIntermediate PackagePackage ¾ In one type of winding operation, strands are collected into an "intermediate" package that is further processed in one of several ways. -

Research on Laser-TIG Hybrid Welding of 6061-T6 Aluminum Alloys Joint and Post Heat Treatment
metals Article Research on Laser-TIG Hybrid Welding of 6061-T6 Aluminum Alloys Joint and Post Heat Treatment Hongyang Wang * , Xiaohong Liu and Liming Liu Key Laboratory of Liaoning Advanced Welding and Joining Technology & School of Materials Science and Engineering, Dalian University of Technology, Dalian 116024, China; [email protected] (X.L.); [email protected] (L.L.) * Correspondence: [email protected] Received: 22 November 2019; Accepted: 7 January 2020; Published: 15 January 2020 Abstract: The 6061-T6 aluminum (Al) alloys was joined by the laser induced tungsten inert gas (TIG) hybrid welding technique. It mainly studied the influences of welding parameters, solution, and aging (STA) treatment on the microstructure and tensile properties of Al alloy hybrid welding joints. Microstructures of the joints were also analyzed by optical microscopy and transmission electron microscopy. Results showed that the laser induced arc hybrid welding source changed the microstructure of the fusion zone and heat effect zone. STA treatment effectively improved the mechanical properties of the softening area in the hybrid welding joint, whose values of the tensile strength and elongation were on average 286 MPa and 20.5%. The distribution of the reinforcement phases and dislocations distributed were more uniform, which improved the property of STA treated joint. Keywords: Al alloy; laser induced arc hybrid welding; heat treatment; microstructure 1. Introduction Aluminum alloys are used as structural materials, due to the high strength, excellent corrosion resistance, and low density, which makes them promising alternatives to steels for lightweight structures such as vehicles and airplanes [1–7]. Aluminum alloys are also extensively used in aviation, vessel, and electronics industries due to their light weight, high specific strength, good corrosion resistance, and excellent mechanical properties [8–16]. -

Fatigue of Ti-6Al-4V
Chapter 3 Fatigue of Ti-6Al-4V Shabnam Hosseini Additional information is available at the end of the chapter http://dx.doi.org/10.5772/45753 1. Introduction Metallic biomaterials have essentially three fields of use; these are the artificial hip joints, screw, plates and nails for internal fixation of fractures, and dental implants. Any of these devices must support high mechanical load and resistance of material against breakage is essential. High mechanical properties are needed for structural efficiency of surgical and dental implants. But their volume is restricted by anatomic realities what require good yield and fatigue strengths of metal [1]. The use of titanium alloys is due to their excellent corrosion resistance. Also, that is because of their tensile strength, a high strength to weight ratio and low elastic modulus. Titanium continues to be widely used in biomedical applications. Ti-6Al-4V alloy is the most frequently used these days [2]. Fatigue fracture and wear have been identified as some of the major problems associated with implant loosening, stress-shielding and ultimate implant failure. Although wear is commonly reported in orthopedic applications such as knee and hip joint prostheses, it is also a serious and often fatal experience in mechanical heart valves. Fig.1 illustrates some examples of fatigue fracture of implant devices in the hip prosthesis and a mechanical heart valve. It can be seen that fatigue-wear interaction plays a significant role in ultimate failure of these medical devices [3]. In orthopedic implants design, it is unavoidable the presence of geometrical fillets such as notches which cause stress locally. -

STUDYING SOME of the MECHANICAL PROPERTIES of UNSATURATED POLYESTER REINFORCED by RE-CYCLED NATURAL MATERIALS Shaimaa Hilal Kamel Assist
Al-Qadisiyah Journal For Engineering Sciences, Vol. 8……No. 2 ….2015 STUDYING SOME OF THE MECHANICAL PROPERTIES OF UNSATURATED POLYESTER REINFORCED BY RE-CYCLED NATURAL MATERIALS Shaimaa Hilal Kamel Assist. Lecturer Mechanical Engineering Department, University of Technology [email protected] Received 15 February 2015 Accepted 6 May 2015 ABSTRACT Reinforcement of unsaturated polyester by particulates plays is an important role in the improvement of the mechanical properties of high performance materials. Hence, the mechanical behavior of recycled (grapes and dates) particulate polyester composites was studied in order to develop an engineering material for industrial applications. Different percentages (0, 1.5, 3.5 and 5) % of the reinforcement Particles were added to unsaturated polyester resin. Numbers of mechanical tests were included (thermal conductivity test, hardness test, Dielectric testing, tensile test, impact test). The results showed that the hardness and dielectric constant of filled unsaturated polyester increase with the increment of the percentage of grapes and dates particles, while impact resistance decreases. On the other hand the thermal conductivity increase at (1.5%) of percentages and decreases of (3.5%, 5%) of grapes particles while the value of thermal conductivity decrease by (1.5%) and increased when increasing (3.5 %, 5%) of the dates particle. The tensile strength improves of grapes reinforcing particles and increase with an increase in the particle percentage of grapes and the value of tensile strength increases with the dates particle content (1.5%). Also that the percentage of (3.5 %) represents the greatest value for the modulus of elasticity for unsaturated polyester reinforced with dates particle and the percentage of (5 %) represents the greatest value for the modulus of elasticity for unsaturated polyester reinforced with grapes particle. -

Comparison of the Mechanical Properties of AL6061/Albite and AL6061/Graphite Metal Matrix Composites
Journal of Minerals & Materials Characterization & Engineering, Vol. 8, No.2, pp 93-106, 2009 jmmce.org Printed in the USA. All rights reserved Comparison of the Mechanical Properties of AL6061/Albite and AL6061/Graphite Metal Matrix Composites A. Ramesh 1, J. N. Prakash 1*, A. S. Shiva Shankare Gowda2 and 3 Sonnappa Appaiah 1Department of Mechanical Engineering, Alpha College of Engineering Bangalore-562149, INDIA 2 Dr.M.G.R Educational and Research Institute, Chennai-600 095, INDIA 3Department of Industrial Engineering and Management, M.S.R.I.T, Bangalore-560 075, INDIA * Corresponding author’s e-mail address: [email protected] ABSTRACT The aim of this present investigation is to carry out a comparative study of the mechanical properties of AL6061/Albite composites containing albite(NaAlSi3O8) particulates, which are naturally occurring plagioclase feldspar and AL6061/graphite particulate composites containing graphite particles. The reinforcing particulates in the MMC’s vary from 0% to 4% by weight. The 'vortex method' of production was employed to fabricate the composites, in which the reinforcements were poured into the vortex created by stirring the molten metal by means of a mechanical agitator. The composites so produced were subjected to a series of tests. The results of this study revealed that as the Albite particle content was increased, there were significant increases in the ultimate tensile strength, hardness and Young's modulus, accompanied by a reduction in its ductility. There was, however, only a very marginal increase in the compressive strength, where as in graphite reinforced composites as the graphite content was increased, there were significant reduction in hardness and monotonic increases in the ductility, ultimate tensile strength (UTS), compressive strength and Young's modulus of the composite, An attempt is made in the paper to provide explanations for these phenomena. -

Template for SPE ACCE Conference
BIO-BASED POLYAMIDES REINFORCED WITH CELLULOSE NANOFIBERS—PROCESSING AND CHARACTERIZATION Jennifer H. Zhu, Alper Kiziltas, Ellen C. Lee, Deborah Mielewski Materials Research and Advanced Engineering, Ford Motor Company, Dearborn, MI, 48124 Abstract Bio-based polyamides are among the most promising families of bioplastics based on fully or partially derived renewable sources because of their low density, good mechanical and thermal properties, and durability. Bio-based polyamides (e.g., PA 11, PA 1010, and, to a lesser extent, PA 610) are also key resins for automotive applications because their continuous operating temperatures are comparable to the widely used PA6 and PA66. In this study, cellulose nanofibers (CNF) have been successfully dispersed in bio-based polyamide matrices (PA610 and PA1010) by conventional melt processing. The effects of CNF contents on the mechanical (tensile, flexural, and impact) and thermal (crystallization behavior and thermal stability) properties were investigated. The results indicate that these CNF fillers can be efficiently incorporated into the bio-based polymer matrices without the need for coupling agents, surface modifications or surfactants. The distribution and dispersion of the particles within the polymer matrix were studied using scanning electron microscopy. The composites produced here using bio-based polyamides have good mechanical and thermal properties and could be especially useful in applications within or near the engine. Introduction Traditional high performance polymer composites have been made in the past using inorganic fillers such as carbon fibers, glass fibers, and talc fillers. Although these composites have good mechanical and thermal properties, they are not renewable and not biodegradable. Current research focuses upon finding natural, environmentally-friendly, and renewable fibers to replace traditional fillers. -
Today's Space Elevator
International Space Elevator Consortium ISEC Position Paper # 2019-1 Today's Space Elevator Space Elevator Matures into the Galactic Harbour A Primer for Progress in Space Elevator Development Peter Swan, Ph.D. Michael Fitzgerald ii Today's Space Elevator Space Elevator Matures into the Galactic Harbour Peter Swan, Ph.D. Michael Fitzgerald Prepared for the International Space Elevator Consortium Chief Architect's Office Sept 2019 iii iv Today's Space Elevator Copyright © 2019 by: Peter Swan Michael Fitzgerald International Space Elevator Consortium All rights reserved, including the rights to reproduce this manuscript or portions thereof in any form. Published by Lulu.com [email protected] 978-0-359-93496-6 Cover Illustrations: Front – with permission of Galactic Harbour Association. Back – with permission of Michael Fitzgerald. Printed in the United States of America v vi Preface The Space Elevator is a Catalyst for Change! There was a moment in time that I realized the baton had changed hands - across three generations. I was talking within a small but enthusiastic group of attendees at the International Space Development Conference in June 2019. On that stage there was generation "co-inventor" Jerome Pearson, generation "advancing concept" Michael Fitzgerald and generation "excited students" James Torla and Souvik Mukherjee. The "moment" was more than an assembly of young and old. It was also a portrait of the stewards of the Space Elevator revolution -- from Inventor to Developer to Innovators. James was working a college research project on how to get to Mars in 77 days from the Apex Anchor and Souvik (16 years old) was representing his high school from India. -

Chapter 16 Composites
1496T_c16_577-620 12/31/05 14:08 Page 577 2nd REVISE PAGES Chapter 16 Composites Top. ABS plastic having Bidirectional layers. 45 a low glass transition temperature. fiberglass. Provide torsional Used for containment and cosmetic stiffness. purposes. Unidirectional layers. 0 (and Side. ABS plastic some 90 ) fiberglass. Provide having a low glass longitudinal stiffness. transition temperature. Containment and cosmetic. Core wrap. Bidirectional layer of fiberglass. Acts as a torsion box and bonds outer layers to core. Core. Polyurethane plastic. Acts as a filler. Bidirectional layer. 45 fiberglass. Damping layer. Polyurethane. Provides torsional Improves chatter resistance. stiffness. Unidirectional layers. 0 (and some 90 ) fiberglass. Provide longitudinal stiffness. Edge. Hardened steel. Facilitates Bidirectional layer. 45 fiberglass. turning by “cutting” Provides torsional stiffness. into the snow. Base. Compressed carbon (carbon particles embedded in a plastic matrix). Hard and abrasion resistant. Provides appropriate surface. One relatively complex composite structure is the modern ski. In this illustration, a cross section of a high-performance snow ski, are shown the various components. The function of each component is noted, as well as the material that is used in its construction. (Courtesy of Evolution Ski Company, Salt Lake City, Utah.) WHY STUDY Composites? With a knowledge of the various types of composites, property combinations that are better than those as well as an understanding of the dependence of found in the metal alloys, ceramics, and polymeric their behaviors on the characteristics, relative amounts, materials. For example, in Design Example 16.1, we geometry/distribution, and properties of the con- discuss how a tubular shaft is designed that meets stituent phases, it is possible to design materials with specified stiffness requirements. -

Effect of Particulate Reinforcements on the Mechanical Properties of Al6061-WC and Al6061-Gr Mmcs
Journal of Minerals & Materials Characterization & Engineering , Vol. 10, No.12, pp.1141-1152, 2011 jmmce.org Printed in the USA. All rights reserved Effect of Particulate Reinforcements on the Mechanical Properties of Al6061-WC and Al6061-Gr MMCs A. R. K. Swamy 1, A. Ramesha 2, G.B. Veeresh Kumar 3* , J. N. Prakash 4 1Research Scholar, Dr. M.G.R Educational & Research Institute, Chennai & Department Mechatronics Engineering, Acharya Institute of Technology, Bangalore, India. 2GATES Institute of Technology, Gooty, Andhra Pradesh, India. 3Department of Mechanical Engineering, Amrita Vishwa Vidyapeetham, Bangalore, India. 4Department of Mechanical Engineering, Alpha College of Engineering, Bangalore , India. *Corresponding author: [email protected] ABSTRACT This paper presents the comparative study of the mechanical properties of Al6061-Tungsten carbide composites containing Tungsten carbide (WC) particulate, and Al6061-graphite particulate composites containing graphite particles. The reinforcing particulates in the MMCs vary from 0% to 4% by weight. The 'vortex method' of production was employed to fabricate the composites, in which the reinforcements were poured into the vortex created by stirring the molten metal by means of a mechanical agitator. The composites so produced were subjected to a series of tests. The results of this study revealed that as the Tungsten carbide particle content was increased, there were significant increases in the ultimate tensile strength, hardness and Young's modulus, accompanied by a reduction in its ductility. There was, however, only a very marginal increase in the compressive strength, where as in graphite reinforced composites as the graphite content was increased, there were significant reduction in hardness and monotonic increases in the ductility, ultimate tensile strength (UTS), compressive strength and Young's modulus of the composite, An attempt is made in the paper to provide explanations for these phenomena.