Case Report Systemic Pseudohypoaldosteronism Type I: a Case Report and Review of the Literature
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Case Study: Testing for Genetic Disorders
Case study: Testing for genetic disorders Purpose: A genetic disorder is a disorder that is caused by an abnormality (or several abnormalities) in a person’s genome. Genetic disorders are often hereditary, which means they are passed down to a child from its parents. The most common genetic disorder is familial hypercholesterolaemia (a genetic predisposition to high cholesterol levels), which is thought to affect 1 in 250 people in the UK. Other disorders, such as cystic fibrosis, are rarer although they can be relatively common in particular ethnic groups; for instance, the incidence of cystic fibrosis in Scotland is almost double the global average. Collectively genetic disorders affect lots of people because there are more than 10,000 different types worldwide. Genetic testing is used to inform people whether they have a disorder, or if there is a chance they might pass a disorder on to their children. Photo credit: jxfzsy/ iStockphoto Photo credit: monkeybusiness/ iStockphoto Approaches to identifying genetic disorders In the UK, if a person is concerned they might have, or might develop, a genetic disorder based on their family history, they can: Go to their GP, who might refer them for genetic testing. Patients usually provide a DNA sample, which is then analysed for a specific abnormality. Usually a single gene will be tested, but whole-genome sequencing approaches are being developed. The 100,000 Genomes Project, for example, aims to study whole-genome sequences from patients with cancer and rare disease Use a commercial genetic testing service. In this case, customers collect samples of their own DNA and send them away for analysis and interpretation Take no action. -

DOI: 10.4274/Jcrpe.Galenos.2021.2020.0175
DOI: 10.4274/jcrpe.galenos.2021.2020.0175 Case report A novel SCNN1A variation in a patient with autosomal-recessive pseudohypoaldosteronism type 1 Mohammed Ayed Huneif1*, Ziyad Hamad AlHazmy2, Anas M. Shoomi 3, Mohammed A. AlGhofely 3, Dr Humariya Heena 5, Aziza M. Mushiba 4, Abdulhamid AlSaheel3 1Pediatric Endocrinologist at Najran university hospital, Najran Saudi Arabia. 2 Pediatric Endocrinologist at Al yamammah hospital, , Riyadh, Saudi Arabia. 3 Pediatric Endocrinologist at Pediatric endocrine department,. Obesity, Endocrine, and Metabolism Center, , King Fahad Medical City, Riyadh, Saudi Arabia. 4Clinical Geneticist, Pediatric Subspecialties Department, Children's Specialized Hospital, King Fahad Medical City, Riyadh, Saudi Arabia. 5 Research Center, King Fahad Medical City, Riyadh , Saudi Arabia What is already known on this topic ? Autosomal-recessive pseudohypoaldosteronism type 1 (PHA1) is a rare genetic disorder caused by different variations in the ENaC subunit genes. Most of these variations appear in SCNN1A mainly in exon eight, which encodes for the alpha subunit of the epithelial sodium channel ENaC. Variations are nonsense, single-base deletions or insertions, or splice site variations, leading to mRNA and proteins of abnormal length. In addition, a few new missense variations have been reported. What this study adds ? We report a novel mutation [ c.729_730delAG (p.Val245Glyfs*65) ] in the exon 4 of the SCNN1A gene In case of autosomal recessive pseudohypoaldosteronism type 1. Patient with PHA1 requires early recognition, proper treatment, and close follow-up. Parents are advised to seek genetic counseling and plan future pregnancies. proof Abstract Pseudohypoaldosteronism type 1 (PHA1) is an autosomal-recessive disorder characterized by defective regulation of body sodium levels. -

Restoration of Epithelial Sodium Channel Function by Synthetic Peptides in Pseudohypoaldosteronism Type 1B Mutants
ORIGINAL RESEARCH published: 24 February 2017 doi: 10.3389/fphar.2017.00085 Restoration of Epithelial Sodium Channel Function by Synthetic Peptides in Pseudohypoaldosteronism Type 1B Mutants Anita Willam 1*, Mohammed Aufy 1, Susan Tzotzos 2, Heinrich Evanzin 1, Sabine Chytracek 1, Sabrina Geppert 1, Bernhard Fischer 2, Hendrik Fischer 2, Helmut Pietschmann 2, Istvan Czikora 3, Rudolf Lucas 3, Rosa Lemmens-Gruber 1 and Waheed Shabbir 1, 2 1 Department of Pharmacology and Toxicology, University of Vienna, Vienna, Austria, 2 APEPTICO GmbH, Vienna, Austria, 3 Vascular Biology Center, Medical College of Georgia, Augusta University, Augusta, GA, USA Edited by: The synthetically produced cyclic peptides solnatide (a.k.a. TIP or AP301) and its Gildas Loussouarn, congener AP318, whose molecular structures mimic the lectin-like domain of human University of Nantes, France tumor necrosis factor (TNF), have been shown to activate the epithelial sodium Reviewed by: channel (ENaC) in various cell- and animal-based studies. Loss-of-ENaC-function Stephan Kellenberger, University of Lausanne, Switzerland leads to a rare, life-threatening, salt-wasting syndrome, pseudohypoaldosteronism type Yoshinori Marunaka, 1B (PHA1B), which presents with failure to thrive, dehydration, low blood pressure, Kyoto Prefectural University of Medicine, Japan anorexia and vomiting; hyperkalemia, hyponatremia and metabolic acidosis suggest *Correspondence: hypoaldosteronism, but plasma aldosterone and renin activity are high. The aim of Anita Willam the present study was to investigate whether the ENaC-activating effect of solnatide [email protected] and AP318 could rescue loss-of-function phenotype of ENaC carrying mutations at + Specialty section: conserved amino acid positions observed to cause PHA1B. -

Inherited Renal Tubulopathies—Challenges and Controversies
G C A T T A C G G C A T genes Review Inherited Renal Tubulopathies—Challenges and Controversies Daniela Iancu 1,* and Emma Ashton 2 1 UCL-Centre for Nephrology, Royal Free Campus, University College London, Rowland Hill Street, London NW3 2PF, UK 2 Rare & Inherited Disease Laboratory, London North Genomic Laboratory Hub, Great Ormond Street Hospital for Children National Health Service Foundation Trust, Levels 4-6 Barclay House 37, Queen Square, London WC1N 3BH, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-2381204172; Fax: +44-020-74726476 Received: 11 February 2020; Accepted: 29 February 2020; Published: 5 March 2020 Abstract: Electrolyte homeostasis is maintained by the kidney through a complex transport function mostly performed by specialized proteins distributed along the renal tubules. Pathogenic variants in the genes encoding these proteins impair this function and have consequences on the whole organism. Establishing a genetic diagnosis in patients with renal tubular dysfunction is a challenging task given the genetic and phenotypic heterogeneity, functional characteristics of the genes involved and the number of yet unknown causes. Part of these difficulties can be overcome by gathering large patient cohorts and applying high-throughput sequencing techniques combined with experimental work to prove functional impact. This approach has led to the identification of a number of genes but also generated controversies about proper interpretation of variants. In this article, we will highlight these challenges and controversies. Keywords: inherited tubulopathies; next generation sequencing; genetic heterogeneity; variant classification. 1. Introduction Mutations in genes that encode transporter proteins in the renal tubule alter kidney capacity to maintain homeostasis and cause diseases recognized under the generic name of inherited tubulopathies. -

Inheriting Genetic Conditions
Help Me Understand Genetics Inheriting Genetic Conditions Reprinted from MedlinePlus Genetics U.S. National Library of Medicine National Institutes of Health Department of Health & Human Services Table of Contents 1 What does it mean if a disorder seems to run in my family? 1 2 Why is it important to know my family health history? 4 3 What are the different ways a genetic condition can be inherited? 6 4 If a genetic disorder runs in my family, what are the chances that my children will have the condition? 15 5 What are reduced penetrance and variable expressivity? 18 6 What do geneticists mean by anticipation? 19 7 What are genomic imprinting and uniparental disomy? 20 8 Are chromosomal disorders inherited? 22 9 Why are some genetic conditions more common in particular ethnic groups? 23 10 What is heritability? 24 Reprinted from MedlinePlus Genetics (https://medlineplus.gov/genetics/) i Inheriting Genetic Conditions 1 What does it mean if a disorder seems to run in my family? A particular disorder might be described as “running in a family” if more than one person in the family has the condition. Some disorders that affect multiple family members are caused by gene variants (also known as mutations), which can be inherited (passed down from parent to child). Other conditions that appear to run in families are not causedby variants in single genes. Instead, environmental factors such as dietary habits, pollutants, or a combination of genetic and environmental factors are responsible for these disorders. It is not always easy to determine whether a condition in a family is inherited. -

Restoration of Fertility by Gonadotropin Replacement in a Man With
J Rohayem and others Fertility in hypogonadotropic 170:4 K11–K17 Case Report CAH with TARTs Restoration of fertility by gonadotropin replacement in a man with hypogonadotropic azoospermia and testicular adrenal rest tumors due to untreated simple virilizing congenital adrenal hyperplasia Julia Rohayem1, Frank Tu¨ ttelmann2, Con Mallidis3, Eberhard Nieschlag1,4, Sabine Kliesch1 and Michael Zitzmann1 Correspondence should be addressed 1Center of Reproductive Medicine and Andrology, Clinical Andrology, University of Muenster, Albert-Schweitzer- to J Rohayem Campus 1, Building D11, D-48149 Muenster, Germany, 2Institute of Human Genetics and 3Institute of Reproductive Email and Regenerative Biology, Center of Reproductive Medicine and Andrology, University of Muenster, Muenster, Julia.Rohayem@ Germany and 4Center of Excellence in Genomic Medicine Research, King Abdulaziz University, Jeddah, Saudi Arabia ukmuenster.de Abstract Context: Classical congenital adrenal hyperplasia (CAH), a genetic disorder characterized by 21-hydroxylase deficiency, impairs male fertility, if insufficiently treated. Patient: A 30-year-old male was referred to our clinic for endocrine and fertility assessment after undergoing unilateral orchiectomy for a suspected testicular tumor. Histopathological evaluation of the removed testis revealed atrophy and testicular adrenal rest tumors (TARTs) and raised the suspicion of underlying CAH. The remaining testis was also atrophic (5 ml) with minor TARTs. Serum 17-hydroxyprogesterone levels were elevated, cortisol levels were at the lower limit of normal range, and gonadotropins at prepubertal levels, but serum testosterone levels were within the normal adult range. Semen analysis revealed azoospermia. CAH was confirmed by a homozygous mutation g.655A/COG (IVS2-13A/COG) in European Journal of Endocrinology CYP21A2. Hydrocortisone (24 mg/m2) administered to suppress ACTH and adrenal androgen overproduction unmasked deficient testicular testosterone production. -

Prevalence and Incidence of Rare Diseases: Bibliographic Data
Number 1 | January 2019 Prevalence and incidence of rare diseases: Bibliographic data Prevalence, incidence or number of published cases listed by diseases (in alphabetical order) www.orpha.net www.orphadata.org If a range of national data is available, the average is Methodology calculated to estimate the worldwide or European prevalence or incidence. When a range of data sources is available, the most Orphanet carries out a systematic survey of literature in recent data source that meets a certain number of quality order to estimate the prevalence and incidence of rare criteria is favoured (registries, meta-analyses, diseases. This study aims to collect new data regarding population-based studies, large cohorts studies). point prevalence, birth prevalence and incidence, and to update already published data according to new For congenital diseases, the prevalence is estimated, so scientific studies or other available data. that: Prevalence = birth prevalence x (patient life This data is presented in the following reports published expectancy/general population life expectancy). biannually: When only incidence data is documented, the prevalence is estimated when possible, so that : • Prevalence, incidence or number of published cases listed by diseases (in alphabetical order); Prevalence = incidence x disease mean duration. • Diseases listed by decreasing prevalence, incidence When neither prevalence nor incidence data is available, or number of published cases; which is the case for very rare diseases, the number of cases or families documented in the medical literature is Data collection provided. A number of different sources are used : Limitations of the study • Registries (RARECARE, EUROCAT, etc) ; The prevalence and incidence data presented in this report are only estimations and cannot be considered to • National/international health institutes and agencies be absolutely correct. -

A New Age in the Genetics of Deafness
, September/October 1999. Vol. 1 . No. 6 invited review/cme article A new age in the genetics of deafness Heidi L. Rehtu, MMSC' ot~dCytltllia C. Morton, PAD' Over the last several years, an understanding of the genetics SYNDROMIC AND NONSYNDROMIC DEAFNESS of hearing and deafness has grown exponentially. This progress , The prevalence of severe to profound bilateral congenital has been fueled by fast-paced developments in gene mapping hearing loss is estimated at 1 in 1000 births? When milder I and discovery, leading to the recent cloning and characteriza- forms of permanent hearing impairment at birth are included, tion of many genes critical in the biology of hearing. Among this estimate increases four-fold. Congenital deafness refers to the most significant advances, especially from a clinical per- deafness present at birth, though the cause of such hearing spective, is the discovery that up to 50% of nonsyndromic re- impairment may be genetic (hereditary) or environmental (ac- cessive deafness is caused by mutations in the GIB2 gene, which quired). Various studies estimate that approximately one-half encodes the connexin 26 protein (Cx26).I.' The clinical use of of the cases of congenital deafness are due to genetic factor^,^ screening for mutations in Cx26 and other genes involved in and these factors can be further subdivided by mode of inher- hearing impairment is still a new concept and not widely avail- itance. Approximately 77% of cases of hereditary deafness are able. However, the eventual availability of an array of genetic autosomal recessive, 22% are autosomal dominant, and 1% are tests is likely to have a major impact on the medical decisions X-linked.-l In addition, a small fraction of hereditary deafness made by hearing-impaired patients, their families, and their (< 1%) represents those families with mitochondria1 inheri- physicians. -

Waardenburg Syndrome: Case Series
International Journal of Otorhinolaryngology and Head and Neck Surgery Sachdeva S et al. Int J Otorhinolaryngol Head Neck Surg. 2021 Apr;7(4):668-671 http://www.ijorl.com pISSN 2454-5929 | eISSN 2454-5937 DOI: https://dx.doi.org/10.18203/issn.2454-5929.ijohns20211191 Case Series Waardenburg syndrome: case series Sheenu Sachdeva*, Varunkumar Jayakumar, Shubhlaxmi Atmaram Jaiswal Department of Otorhinolaryngology, Dr. V. M. G. M. C Solapur, Maharashtra, India Received: 13 January 2021 Revised: 16 February 2021 Accepted: 06 March 2021 *Correspondence: Dr. Sheenu Sachdeva, E-mail: [email protected] Copyright: © the author(s), publisher and licensee Medip Academy. This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited. ABSTRACT Waardenburg syndrome is a rare genetic disorder of neural crest cell development with incidence of 1:42000 to 1:50,000. The syndrome is not completely expressed and hence adds to its hetergenecity with symptoms varying from one type of syndrome to another and from one patient to another. Unilateral heterochromia that manifests in some people is associated with Waardenburg syndrome and Parry-Romberg syndrome. This is a case series of four cases with features of Waardenburg syndrome with variable presentations and familial inheritance. Keywords: Autosomal dominant deafness, Heterochromia, Pigmentation anomalies, Waardenburg syndrome INTRODUCTION normal. Her mother also had similar hypertelorism with normal hearing (Figure 2). Also there is history suggestive Waardenburg syndrome (WS) is a rare genetic disorder of of premature graying of hair in her grandmother since the neural crest development characterized by varying degrees age of 15 years. -

Insurance and Advance Pay Test Requisition
Insurance and Advance Pay Test Requisition (2021) For Specimen Collection Service, Please Fax this Test Requisition to 1.610.271.6085 Client Services is available Monday through Friday from 8:30 AM to 9:00 PM EST at 1.800.394.4493, option 2 Patient Information Patient Name Patient ID# (if available) Date of Birth Sex designated at birth: 9 Male 9 Female Street address City, State, Zip Mobile phone #1 Other Phone #2 Patient email Language spoken if other than English Test and Specimen Information Consult test list for test code and name Test Code: Test Name: Test Code: Test Name: 9 Check if more than 2 tests are ordered. Additional tests should be checked off within the test list ICD-10 Codes (required for billing insurance): Clinical diagnosis: Age at Initial Presentation: Ancestral Background (check all that apply): 9 African 9 Asian: East 9 Asian: Southeast 9 Central/South American 9 Hispanic 9 Native American 9 Ashkenazi Jewish 9 Asian: Indian 9 Caribbean 9 European 9 Middle Eastern 9 Pacific Islander Other: Indications for genetic testing (please check one): 9 Diagnostic (symptomatic) 9 Predictive (asymptomatic) 9 Prenatal* 9 Carrier 9 Family testing/single site Relationship to Proband: If performed at Athena, provide relative’s accession # . If performed at another lab, a copy of the relative’s report is required. Please attach detailed medical records and family history information Specimen Type: Date sample obtained: __________ /__________ /__________ 9 Whole Blood 9 Serum 9 CSF 9 Muscle 9 CVS: Cultured 9 Amniotic Fluid: Cultured 9 Saliva (Not available for all tests) 9 DNA** - tissue source: Concentration ug/ml Was DNA extracted at a CLIA-certified laboratory or a laboratory meeting equivalent requirements (as determined by CAP and/or CMS)? 9 Yes 9 No 9 Other*: If not collected same day as shipped, how was sample stored? 9 Room temp 9 Refrigerated 9 Frozen (-20) 9 Frozen (-80) History of blood transfusion? 9 Yes 9 No Most recent transfusion: __________ /__________ /__________ *Please contact us at 1.800.394.4493, option 2 prior to sending specimens. -

Hearing Loss in Waardenburg Syndrome: a Systematic Review
Clin Genet 2016: 89: 416–425 © 2015 John Wiley & Sons A/S. Printed in Singapore. All rights reserved Published by John Wiley & Sons Ltd CLINICAL GENETICS doi: 10.1111/cge.12631 Review Hearing loss in Waardenburg syndrome: a systematic review Song J., Feng Y., Acke F.R., Coucke P., Vleminckx K., Dhooge I.J. Hearing J. Songa,Y.Fenga, F.R. Ackeb, loss in Waardenburg syndrome: a systematic review. P. Couckec,K.Vleminckxc,d Clin Genet 2016: 89: 416–425. © John Wiley & Sons A/S. Published by and I.J. Dhoogeb John Wiley & Sons Ltd, 2015 aDepartment of Otolaryngology, Xiangya Waardenburg syndrome (WS) is a rare genetic disorder characterized by Hospital, Central South University, Changsha, People’s Republic of China, hearing loss (HL) and pigment disturbances of hair, skin and iris. b Classifications exist based on phenotype and genotype. The auditory Department of Otorhinolaryngology, cDepartment of Medical Genetics, Ghent phenotype is inconsistently reported among the different Waardenburg types University/Ghent University Hospital, and causal genes, urging the need for an up-to-date literature overview on Ghent, Belgium, and dDepartment for this particular topic. We performed a systematic review in search for articles Biomedical Molecular Biology, Ghent describing auditory features in WS patients along with the associated University, Ghent, Belgium genotype. Prevalences of HL were calculated and correlated with the different types and genes of WS. Seventy-three articles were included, describing 417 individual patients. HL was found in 71.0% and was Key words: genotype – hearing loss – predominantly bilateral and sensorineural. Prevalence of HL among the inner ear malformation – phenotype – different clinical types significantly differed (WS1: 52.3%, WS2: 91.6%, Waardenburg syndrome WS3: 57.1%, WS4: 83.5%). -
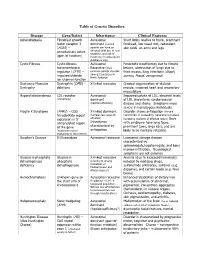
Table of Genetic Disorders Disease Gene/Defect Inheritance Clinical
Table of Genetic Disorders Disease Gene/Defect Inheritance Clinical Features Achondroplasia Fibroblast growth Autosomal Short limbs relative to trunk, prominent factor receptor 3 dominant (normal forehead, low nasal root, redundant (FGR3) – parents can have an skin folds on arms and legs constitutively active affected child due to new mutation, and risk of (gain of function) recurrence in subsequent children is low) Cystic Fibrosis Cystic fibrosis Autosomal Pancreatic insufficiency due to fibrotic transmembrane Recessive (most lesions, obstruction of lungs due to regulator (CFTR) – common genetic disorder thick mucus, lung infections (Staph, impaired chloride among Caucasians in aureus, Pseud. aeruginosa) North America) ion channel function Duchenne Muscular Dystrophin (DMD) - X-linked recessive Gradual degeneration of skeletal Dystrophy deletions muscle, impaired heart and respiratory musculature Hypercholesterolemia LDL receptor Autosomal Impaired uptake of LDL, elevated levels (commonly) dominant of LDL cholesterol, cardiovascular (haploinsufficiency) disease and stroke. Symptoms more severe in homozygous individuals Fragile X Syndrome (FMR1) – CGG X-linked dominant Disorder shows anticipation (female trinucleotide repeat (females less severely transmitters in succeeding generations produce expansion in 5’ affected) increasing numbers of affected males) Boys untranslated region Inheritance with syndrome have long faces, of the gene characterized by prominent jaws, large ears, and are (expansion occurs anticipation likely to be mentally retarded. exclusively in the mother) Gaucher’s Disease Β-Glucosidase Autosomal recessive Lysosomal storage disease characterized by splenomegaly,hepatomegaly, and bone marrow infiltration. Neurological symptoms are not common Glucose 6-phosphate Glucose 6- X-linked recessive Anemia (due to increased hemolysis) dehydrogenase phosphate (prominent among induced by oxidizing drugs, deficiency dehydrogenase individuals of sulfonamide antibiotics, sulfones (e.g.