The Top 10 Medtech States
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Patient-Monitoring Systems
17 Patient-Monitoring Systems REED M. GARDNER AND M. MICHAEL SHABOT After reading this chapter,1 you should know the answers to these questions: ● What is patient monitoring, and why is it done? ● What are the primary applications of computerized patient-monitoring systems in the intensive-care unit? ● How do computer-based patient monitors aid health professionals in collecting, analyzing, and displaying data? ● What are the advantages of using microprocessors in bedside monitors? ● What are the important issues for collecting high-quality data either automatically or manually in the intensive-care unit? ● Why is integration of data from many sources in the hospital necessary if a computer is to assist in critical-care-management decisions? 17.1 What Is Patient Monitoring? Continuous measurement of patient parameters such as heart rate and rhythm, respira- tory rate, blood pressure, blood-oxygen saturation, and many other parameters have become a common feature of the care of critically ill patients. When accurate and imme- diate decision-making is crucial for effective patient care, electronic monitors frequently are used to collect and display physiological data. Increasingly, such data are collected using non-invasive sensors from less seriously ill patients in a hospital’s medical-surgical units, labor and delivery suites, nursing homes, or patients’ own homes to detect unex- pected life-threatening conditions or to record routine but required data efficiently. We usually think of a patient monitor as something that watches for—and warns against—serious or life-threatening events in patients, critically ill or otherwise. Patient monitoring can be rigorously defined as “repeated or continuous observations or meas- urements of the patient, his or her physiological function, and the function of life sup- port equipment, for the purpose of guiding management decisions, including when to make therapeutic interventions, and assessment of those interventions” (Hudson, 1985, p. -

Dec 3 1 2013
X 133704. DEC 3 1 2013 510(K) Summary for M1 Capnography Mask 1. Submission Sponsor Monitor Mask, Inc. 551543 dAvenue NE Seattle WA, 98105 USA Phone: 877.226.1776 Contact: JW Beard MD, MBA, Founder and CEO 2. Submission Correspondent Emergo, Group, Inc. 816 Congress Avenue, Suite 1400 Austin, TX 78701 office Phone: (512) 327.9997 Fax: (512) 327.9998 Contact: Stuart RGoldman, Senior Consultant, RA/QA Email: oroiect.manapement~emerpogroup.com 3. Date Prepared September 20, 2013 4. Device Identification Trade/Proprietary Name: M1 Capnography Mask Common/Usual Name: oxygen/capnography mask Classification Name: analyzer, gas, carbon-dioxide, gaseous-phase Classification Regulation: §868.1400 Product Code: CCK (primary); BYG (secondary) Device Class: Class 11 Classification Panel: Anesthesiology 5. Legally Marketed Predicate Device CapnoxygenO Mask (K(971229) manufactured by Medlsys (Southmedic) 6. Device Description The M1 Capnography Mask is connected to a gas source for delivering low flow oxygen through flexible tubing to the patient, and at the same time provides a means to attach a capnograph for monitoring exhaled carbon dioxide during nose and mouth breathing. The capnograph gas sample tube attaches to a standard female Luer connector on either the left or right side of the M1 Capnography Mask. The M1 Capnography Mask will initially be made available in only one size, adult large (Model #001). Page 5-1 7. Indication for Use Statement The M1 Capnography Mask is a single-use device intended for delivering supplemental oxygen and monitoring exhaled carbon dioxide in non-intubated spontaneously breathing patients. Standard oxygen tubing and two female Luer ports for gas sample line attachment are included. -

(2) January 2020 CVJ.Pmd
DOI: https://doi.org/10.3329/cardio.v12i2.47994 Special Article Clarence Walton Lillehei: The Pioneer Open Heart Surgeon Md Anisuzzaman, Nazmul Hosain Department of Cardiac Surgery, Chattogram Medical College Key Words : (Cardiovasc. j. 2020; 12(2): 145-148) Walton Lillehei, Open heart surgery. Introduction: Wangensteen provided a creative environment that Heart as an organ was always a difficult territory produced many brilliant surgeons, and he took a for the surgeons. Famous surgeon Stephen Paget special interest in young Lillehei. in 1895 wrote, “Surgery of the heart has probably In 1949, Dr. Lillehei was appointed a fulltime reached the limit set by the nature, no new instructor of surgery at the University of methods, and no new discovery can overcome the Minnesota Medical School. He began to work his natural difficulties that attend a wound of the way up the academic ladder towards a clinical heart”. But surgeons like Axel Cappelen of Norway professorship. The following year, however fate in 1895, Ludwig Rehn of Germany in 1896 did the dealt him a cruel blow: he was diagnosed with job very well by repairing stab injuries of the heart. lymphosarcoma of the parotid gland and was given But these were rare achievements. Virtually there at most, a 10% chance of surviving for 5 years. was no safe surgical approach or method to deal The day after he finished his senior residency, the heart well before the 1950s. C W Lillehei and Lillehei underwent extensive head and neck his colleagues brought about these changes to surgery at the hands of Dr. Wangensteen and Dr. -

Serious Adverse Event Reporting Under Directives 90/385/Eec and 93/42/Eec
EUROPEAN COMMISSION DG Internal Market, Industry, Entrepreneurship and SMEs Consumer, Environmental and Health Technologies Health technology and Cosmetics MEDDEV 2.7/3 revision 3 May 2015 GUIDELINES ON MEDICAL DEVICES CLINICAL INVESTIGATIONS: SERIOUS ADVERSE EVENT REPORTING UNDER DIRECTIVES 90/385/EEC AND 93/42/EEC. Note The present Guidelines are part of a set of Guidelines relating to questions of application of EC-Directives on medical Devices. They are legally not binding. The Guidelines have been carefully drafted through a process of intensive consultation of the various interested parties (competent authorities, Commission services, industries, other interested parties) during which intermediate drafts where circulated and comments were taken up in the document. Therefore, this document reflects positions taken by representatives of interest parties in the medical devices sector. These guidelines incorporate changes introduced by Directive 2007/47/EC amending Council Directive 90/385/EEC and Council Directive 93/42/EEC. 1 MEDICAL DEVICES DIRECTIVES CLINICAL INVESTIGATION GUIDELINES FOR ADVERSE EVENT REPORTING UNDER DIRECTIVES 90/385/EEC AND 93/42/EEC Index 1. INTRODUCTION 2. SCOPE 3. DEFINITIONS 4. REPORTABLE EVENTS 5. REPORT BY WHOM 6. REPORT TO WHOM 7. REPORTING TIMELINES 8. CAUSALITY ASSESSMENT 9. REPORTING FORM Appendix – Summary Reporting Form 2 1. INTRODUCTION This guidance defines Serious Adverse Event (SAE) reporting modalities and includes a summary tabulation reporting format. Individual reporting should be performed in accordance with national requirements. The objective of this guidance is to contribute to the notification of SAEs to all concerned National Competent Authorities (NCAs ) 1 in the context of clinical investigations in line with the requirements of Annex 7 of Directive 90/385/EEC and Annex X of Directive 93/42/EEC, as amended by Directive 2007/47/EC. -

EIR) Expertise Guide
v MEDICAL INDUSTRY LEADERSHIP INSTITUTE 2019–2020 Executive in Residence (EIR) Expertise Guide John J. Alexander, ChEng, MDA [email protected] President, Business Development Advisors, Inc.; Chairman, Twin Cities Angels Funds Roles: Corporate Development, CEO, Management Team Coach Background: St. Jude Medical, Baxter Healthcare, W.R. Grace, GE, Alcoa. Private companies—Wide Range Expertise: (Markets) Medical Technology: devices, diagnostics (Functions) Corporate Development: M&A, strategic alliances, divestitures, licensing, corporate ventures, internal startups starting, building emerging technology businesses and turning-around established businesses; (Functions) venture capital & angel capital, PIPE, venture debt, debt Susan Alpert, PhD, MD [email protected] Principal, SFA Consulting Roles: Regulatory affairs, device evaluation, pediatrician with specialty in infectious disease Background: Medtronic, C.R. Bard, FDA Expertise: Regulatory affairs, FDA, clinical trials, medical devices, business strategy Daniel L. Cosentino, MBA [email protected] CEO, ElectroSea ROLES: CEO, Entrepreneur, Board Advisor, Investor BACKGROUND: Founder & CEO, Cardiocom; VP/GM, Medtronic; Board Advisor, Minnetronix Neuro EXPERTISE: Digital health, medical devices, entrepreneurship, new product development, general management Michael Finch, PhD [email protected] Research Manager, Children’s Hospital MN Roles: Faculty, instructor, research director Background: Carlson School of Management, Children’s Hospital, School of Public Health, UMN, UnitedHealth Group Expertise: Cost, quality and financing of health care in public and private market, research methods and evaluation, valuation of medical innovations, hospital safety, role of physician communication in health care Paul J. Gam, MBA, CGMA, CPA (Inactive) [email protected] Founder and CEO, Zurich Medical Roles: Various management and advisory roles Background: Founder and CEO, Zurich Medical; Board Member, Blue Cross Blue Shield of MN; Partner, Grace Associates; VP International Development, St. -

Executive Compensation 14 ITEM 12
Table of Contents UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, DC 20549 FORM 10-K/A (Amendment No. 1) ☑ Annual Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the Fiscal Year Ended December 31, 2016 OR ☐ Transition Report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the transition period from to . Commission file number 333-199861 MYLAN N.V. (Exact name of registrant as specified in its charter) The Netherlands 98-1189497 (State or other jurisdiction of incorporation or organization) (I.R.S. Employer Identification No.) Building 4, Trident Place, Mosquito Way, Hatfield, Hertfordshire, AL10 9UL, England (Address of principal executive offices) +44 (0) 1707 853 000 (Registrant’s telephone number, including area code) Securities registered pursuant to Section 12(b) of the Act: Title of Each Class: Name of Each Exchange on Which Registered: Ordinary shares, nominal value €0.01 The NASDAQ Stock Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☑ No ☐ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Medical Device Software Regulation Software Qualification And
AHWP/WG1/R001:2014 REFERENCE DOCUMENT (DRAFT for Public Comments) Title: White Paper on Medical Device Software Regulation – Software Qualification and Classification Author: Work Group 1, Pre-Market Submission and CSDT Date 22 May 2014 Copyright © 2014 by the Asian Harmonization Working Party Table of Contents 1. Introduction ............................................................................................................................. 2 2. Definitions ................................................................................................................................ 2 3. Types of Software that are Regulated as Medical Devices ........................................... 4 4. Forms of Medical Device Software .................................................................................... 4 5. SaMD Medical Device Software Classification Globally .............................................. 6 5.1. International Medical Device Regulatory Forum (IMDRF) .................................... 6 5.1.1. IMDRF qualification criteria .......................................................................................... 6 5.2. Australia TGA ..................................................................................................................... 7 5.2.2. Risk classification of software in TGA ...................................................................... 7 5.3. China .................................................................................................................................... -

Ministry of Medical Services Pharmacy and Poisons Board Kenya
MINISTRY OF MEDICAL SERVICES PHARMACY AND POISONS BOARD KENYA GUIDELINES ON SUBMISSION OF DOCUMENTATION FOR REGISTRATION OF MEDICAL DEVICES FIRST EDITION September, 2011 ACKNOWLEDGEMENTS PREFACE Medical Device Regulation in Kenya will be supervised and directed by Kenya Pharmacy and Poisons Board (PPB). Classification, requirements and evaluation of Medical Devices will be mainly simulation of rules and regulations recognized by the international regulatory benchmarks, which are mainly: a. The Pharmacy and Poisons Act Chapter 244 of 2002 b. Global Harmonization Task Force (GHTF) for Medical Device c. EU Medical Device Directives 93/42/EEC, EU In Vitro Diagnostic Device Directive Ministry of Medical Services Page 2 of 2 Pharmacy and Poisons Board Kenya (IVDD) 98/79/EC and EU Active Implantable Medical Device Directive (AIMDD) 90/385/EEC. d. US FDA (United States Food & Drug Administration) e. Australia TGA (Therapeutics Goods Act) The regulation of medical devices in Kenya is aimed at maintaining balance between ensuring product safety, quality and effectiveness and providing the public with timely access to medical devices and preventing the entrance of unsafe or ineffective devices into the market. SCOPE These guidelines shall apply to medical devices and their accessories. For the purposes of these guidelines, accessories shall be treated as medical devices in their own right. Where a device is intended to administer a medicinal product, that device shall be governed by this guideline, without prejudice to the corresponding regulations for registration of medicinal products for human use set by the PPB. If, however, such a device is placed on the market in such a way that the device and the medicinal product form a single integral product which is intended exclusively for use in the given combination and which is not reusable, that single product shall be governed by corresponding regulations for registration of medicinal products for human use set by the PPB. -

Data Protection on Pharmacovigilance and Medical Device Safety
DATA PROTECTION ON PHARMACOVIGILANCE AND MEDICAL DEVICE SAFETY General Galena Pharma Oy, founded in 1996, is a pharmaceutical company specialising in contract manufacturing and packaging. A significant part of the service includes product development, registration & marketing authorization and post-market surveillance. The wide range of our products comprises human and veterinary medicinal products, medical devices in Classes IIa and IIb, cosmetics, food supplements, animal care products and nutritional supplements. The products we manufacture are sold mainly in pharmacies. Galena Pharma has a legal obligation to monitor the adverse events and the effects of their products in the countries where they are getting sold and carry out a continuous risk-benefit assessment. It is called pharmacovigilance and medical device safety operation. With the help of this system and quality assurance management, Galena Pharma can manage adverse events and protect public health. Thanks to this, the safety of Galena Pharma's products is guaranteed. It is the responsibility of the competent authority to inspect and evaluate the pharmacovigilance and medical device safety activities of the company and validate it. Pharmacovigilance and medical device safety obligate Galena Pharma to process the information of the patient and/or the notifier of the adverse reaction, based on which a person can be directly or indirectly identified. These are personal data whose processing is mandatory for Galena Pharma in order to comply with its pharmacovigilance and medical device safety obligations and report any adverse reactions or events that come to their notice to the competent authorities in a relevant and appropriate manner. This privacy statement describes how personal data gets processed for pharmacovigilance and medical device safety purposes under applicable data protection laws and the obligations set out in the EU Data Protection Regulation (EU 2016/679). -
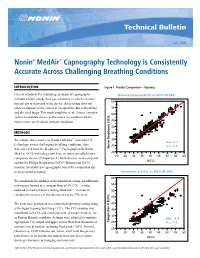
Nonin® Medair™ Capnography Technology Is Consistently Accurate Across Challenging Breathing Conditions
Technical Bulletin July 2009 Nonin® MedAir™ Capnography Technology is Consistently Accurate Across Challenging Breathing Conditions INTRODUCTION Figure 1: Product Comparison – Accuracy Current standards for evaluating accuracy of capnographs NoninNonin RespSense RespSense EtCOEtCO2 vs NICONICO (N-364)(N=364) includes simple, steady flow gas validation in which a known 60 percent gas is delivered to the device. Such testing does not allow evaluation of the effects of complexities due to breathing 55 and diseased lungs. This study sought to create a more complex 2 50 system to evaluate device performance in conditions which better represented realistic patient conditions. 45 40 METHODS 35 Resp EtCO2 To evaluate the accuracy of Nonin’s MedAir™ end-tidal CO 30 2 Bias: 0.2 technology across challenging breathing conditions, data 25 Arms: 2.6 was collected from the RespSense™ Capnograph with Nonin Nonin RespSense EtCO 20 MedAir EtCO2 technology and from an internationally known 20 25 30 35 40 45 50 55 60 competitor device (Competitor A). Both devices were compared ® NICO against the Philips Respironics NICO Mainstream EtCO2 monitor, a mainstream capnograph chosen for comparison due Root Mean Square Error 2.563916 Mean of Response 0.178683 to its accepted accuracy. CompetitorNellcor EtCO A EtCO2 vs2 NICOvs NICO (N=364) (N-364) 60 To compliment the findings of the simulation testing, an additional 55 test was performed at a constant flow of 5% CO2—a value required in medical device testing standards1—in order to 50 validate the accuracy of the devices at a static CO2 level. 2 45 The tests were performed in a controlled laboratory setting using 40 a Michigan Training Test Lung (TTL). -

Final Programme CONTENT
48th EDTNA/ERCA International Conference September 14th-17th, 2019 Prague, Czech Republic Conference Theme New Pathways in the Renal Setting-Caring Together by Integrating Modern Technology based on Knowledge & Education Final Programme CONTENT Welcome Message from the President 3 Acknowledgement – Corporate Members 4 Acknowledgement – Conference Support 5 EDTNA/ERCA Booth 6 Conference Accreditation 6 The Conference Application – Instructions and Logistics 7 Conference Venue – Floor plans 8 Programme at a Glance 9 Scientific Programme Highlights 11 Scientific Programme 14 List of E-Posters 24 The Exhibition Floor Plan 30 The Exhibitors 31 Practical Conference Information 36 Opening Hours Summary 38 Good to know about Prague 38 Conference Contacts 39 2 WELCOME MESSAGE FROM THE PRESIDENT Dear Colleagues, We are very happy to welcome you to Prague for the 48th EDTNA/ERCA Conference. Last time the Conference was held in Prague was in 2008, now, 11 years later we are back in Prague. The Scientific Programme is well prepared and there will be several Workshops, Educational Sessions and Poster sessions. New this year are the Courses which will be interactive sessions allowing everybody to share their expertise. We are, as an Association, in the forefront of Online Communication and it has been a focus for some years and will continue to be one of our focuses for the coming years. Take the opportunity to download the Conference App. It works both for iPhones and Androids. We should never forget that face to face meetings gives us so much more than just to be in a Video call. It has an important role of educating, exchanging information and experience, utilizing the expertise of all of us i.e. -

Connectivity. Access to the Data You Need
CONNECTIVITY. ACCESS TO THE DATA YOU NEED. Integrate across systems with INVOS™5100C cerebral/ somatic oximetry and BIS™ brain monitoring system. We understand your need for easy access to critical patient Connect to patient monitoring systems for better information — and that requires connectivity to standardized visibility of comprehensive patient data electronic medical record (EMR) systems. Our connectivity ™ ™ ™ solutions integrate vital patient data from INVOS cerebral/ INVOS and BIS monitoring systems connect to a variety ™ of multi-parameter monitors, such as Philips patient somatic oximetry and BIS brain monitoring systems into a ™* ™* variety of clinical information and EMR systems. monitoring systems, using Philips VueLink or IntelliBridge interface solutions. Connect to EMR systems for integrated patient data Additional patient monitoring systems compatible with ™ ™ INVOS™ and BIS™ patient monitoring systems are compatible INVOS and/or BIS monitoring systems include: with most EMR and third-party middleware integration systems INVOS™ cerebral/somatic oximetry system in use today, such as: ∙ Philips EMR ∙ Drägerwerk AG & Co. KGaA ∙ Olympus ∙ Cerner ∙ Nihon Kohden ∙ Surgical Information Systems ∙ Epic ∙ Mindray ∙ SAFERsleep ∙ McKesson ∙ Fukuda Denshi ∙ Siemens ∙ AGFR Health Care ∙ Hewlett Packard Theronyz ∙ ORBIS ∙ ™* ∙ Agilent ∙ Philips VISICU ∙ Allscripts ™ ™* BIS brain monitoring system Philips Emergin ∙ Daintel ∙ Middleware ∙ Philips ∙ DocuSys ∙ Nanthealth (iSirona) ∙ Drägerwerk AG & Co. KGaA ∙ Emergisoft ∙ Nihon Kohden General Electric ∙ Capsule Tech ∙ ∙ GE Healthcare ∙ Meditech ∙ Fukuda Denshi Connect to information management systems for Connect to VGA monitors for simultaneous viewing comprehensive clinical records A VGA port on the INVOS™ monitoring system allows the Clinical data management systems such as Anesthesia INVOS™ monitor screen to be displayed on any external Information Management Systems (AIMS), Clinical VGA monitor.