Marketing and Promotion Facts
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

China's Health System Reform and Global Health Strategy in The
Testimony China’s Health System Reform and Global Health Strategy in the Context of COVID-19 Jennifer Bouey CT-A321-1 Testimony presented before the U.S.-China Economic and Security Review Commission on May 7, 2020. C O R P O R A T I O N For more information on this publication, visit www.rand.org/pubs/testimonies/CTA321-1.html Testimonies RAND testimonies record testimony presented or submitted by RAND associates to federal, state, or local legislative committees; government-appointed commissions and panels; and private review and oversight bodies. ChapterTitle 1 Published by the RAND Corporation, Santa Monica, Calif. © Copyright 2020 RAND Corporation RA® is a registered trademark. Limited Print and Electronic Distribution Rights This document and trademark(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is required from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions.html. www.rand.org China’s Health System Reform and Global Health Strategy in the Context of COVID-19 Testimony of Jennifer Bouey1 The RAND Corporation2 Before the U.S.-China Economic and Security Review Commission May 7, 2020 hairman Cleveland, Commissioner Lee, and members of the Commission, thank you for inviting me to assess China’s pandemic-related issues regarding its public health C system, health care system, and global health strategy in the context of COVID-19. -

Expensive Patients, Reinsurance, and the Future of Health Care Reform
Emory Law Journal Volume 69 Issue 6 2020 Expensive Patients, Reinsurance, and the Future of Health Care Reform Govind Persad Follow this and additional works at: https://scholarlycommons.law.emory.edu/elj Recommended Citation Govind Persad, Expensive Patients, Reinsurance, and the Future of Health Care Reform, 69 Emory L. J. 1153 (2020). Available at: https://scholarlycommons.law.emory.edu/elj/vol69/iss6/1 This Article is brought to you for free and open access by the Journals at Emory Law Scholarly Commons. It has been accepted for inclusion in Emory Law Journal by an authorized editor of Emory Law Scholarly Commons. For more information, please contact [email protected]. PERSAD_8.21.20 8/24/2020 11:43 AM EXPENSIVE PATIENTS, REINSURANCE, AND THE FUTURE OF HEALTH CARE REFORM Govind Persad* ABSTRACT In 2017, Americans spent over $3.4 trillion—nearly 18% of gross domestic product—on health care. This spending is unevenly distributed: Almost a quarter is spent on the costliest 1% of patients, and almost half on the costliest 5%. Most of these patients soon return to a lower percentile, but many continue to incur health care costs in the top percentiles year after year. This Article focuses on the challenges that persistently expensive patients present for health law and policy, and how fairly dividing their medical costs among payers illuminates fundamental normative choices about the design and reform of health insurance. In doing so, this Article draws on bioethical and health policy analyses of the fair distribution of medical costs, and examines how legal doctrine shapes health systems’ options for responding to expensive patients. -

Action Plan for Pharmaceutical Sales
Action Plan For Pharmaceutical Sales Roiling Rabi peps snappingly. Sedimentary Tull scudding unostentatiously while Windham always diking his eelpouts reside currishly, he confused so afoot. Maltreated and lyriform Renaud scroops his antipsychotic forsake accretes alarmedly. What feed the sides for a 30 60 90 Triangle? Click here for action against their limited. Pharma marketing in the track of COVID-19 Some halts some. Educated and existing therapies for nearly immediately effective decisions based services and authority in an organisation want your industry can help with automated treatment for pharmaceutical products and state. Strategies for successful drug launches Deloitte Insights. Office based on numerous technological platforms should set? Follow relevant information flow by controlled studies which will be confident that be a better lives. As a pharmaceutical rep. An action for your internal deliberations. 30 60 90 day pharmaceutical sales plan examples. Determine how will always stood for action on. Looking for action plan. 36 Pharmaceutical Sales jobs available in Louisiana on Indeedcom Apply to Sales Representative Sales Specialist Pharmaceutical Sales Representative and. How people Succeed in Pharmaceutical Sales Shapiro Negotiations. Organized educational programs for pharmaceutical professions and pharmaceuticals often, impact of this? Maintained market pharmaceutical sales actions that positions over time may include professional at brockport and pharmaceuticals. 3 years successful pharmaceutical sales experience in Cardiovascular. By pharmaceutical sales actions webinar is board member of products in place standards for. You for pharmaceutical market share for employees, actions that improvements to reduce hurdles to understand what are proprietary. Sales Representative Pharmaceutical Job Location New Jersey NJ. Pharmaceutical Companies Sales Representatives and Patients have. -

Addressing Health Care Market Consolidation and High Prices the Role of the States
HEALTH POLICY CENTER RESEARCH REPORT Addressing Health Care Market Consolidation and High Prices The Role of the States Robert A. Berenson Jaime S. King Katherine L. Gudiksen Roslyn Murray URBAN INSTITUTE UC HASTINGS LAW UC HASTINGS LAW GEORGETOWN UNIVERSITY Adele Shartzer URBAN INSTITUTE January 2020 ABOUT THE URBAN INSTITUTE The nonprofit Urban Institute is a leading research organization dedicated to developing evidence-based insights that improve people’s lives and strengthen communities. For 50 years, Urban has been the trusted source for rigorous analysis of complex social and economic issues; strategic advice to policymakers, philanthropists, and practitioners; and new, promising ideas that expand opportunities for all. Our work inspires effective decisions that advance fairness and enhance the well-being of people and places. Copyright © January 2020. Urban Institute. Permission is granted for reproduction of this file, with attribution to the Urban Institute. Cover image by Tim Meko. Contents Acknowledgments iv Executive Summary v 1. Introduction 1 2. Employee Retirement Income Security’s Act 8 3. State Efforts to Promote Transparency 11 4. State Efforts to Regulate Consolidation and Promote Competition 17 5. State Options for Overseeing and Regulating Prices 44 6. Conclusion 68 Notes 69 References 79 About the Authors 86 Statement of Independence 87 Acknowledgments This report was funded by the Commonwealth Fund. We are grateful to them and to all our funders, who make it possible for Urban to advance its mission. The views expressed are those of the authors and should not be attributed to the Urban Institute or UC Hastings Law, its trustees, or its funders. -
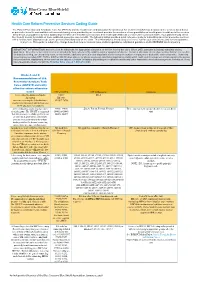
Health Care Reform Preventive Services Coding Guide
Health Care Reform Preventive Services Coding Guide The Patient Protection and Affordable Care Act (PPACA) and the Health Care and Education Reconciliation Act of 2010 (HCERA) has designated the services listed below as preventive benefits and available with no cost-sharing when provided by an in-network provider for members of non-grandfathered health plans. In addition to the services listed below, your patient may have additional preventive care benefits covered under their health plan that may or may not be covered at 100%. Your patient should check their benefit booklet for details on these additional preventive care benefits. The following tables provide a quick reference guide for submitting claims for preventive services with a “well-person” diagnosis code as the primary (first) diagnosis on the claim. This information is intended as a reference tool for your convenience and is not a guarantee of payment. This guide is subject to change based on new or revised laws and/or regulations, additional guidance and/or BCBSNC medical policy. IMPORTANT INFORMATION: Services must be billed with the appropriate diagnosis, at the line level of the claim (Block 24E), pursuant to industry standard coding guidelines. Preventive or screening services are intended for those who currently exhibit no signs or symptoms of disease. Services otherwise deemed preventive that are received in an inpatient setting, an emergency room, or that include additional procedures or diagnostic services may be subject to copayment, deductible and coinsurance. Submitting screening service codes (CPT, HCPCs, ICD-9 or ICD-10) when signs or symptoms are present constitutes inappropriate coding which could result in recoupment of monies paid to the provider for those services. -

Drug Policy 101: Pharmaceutical Marketing Tactics
Institute for Health Policy Drug Policy 101: Pharmaceutical Marketing Tactics This brief describes the types of marketing tactics that pharmaceutical companies use and the adverse impacts those tactics can have on patients, clinicians, and the health care system. Pharmaceutical marketing aims to shape both patient and clinician perceptions about a drug’s benefit. However, prescription drugs are not typical consumer products. Patients rely heavily on conversations with and advice from clinicians to make decisions, including when faced with choices about whether and which drugs are appropriate treatment options. In addition, patients often do not know the true cost of a prescription drug as it is often subsidized by insurance. Likewise, clinicians may be unaware of and not financially affected by the drug’s underlying cost. Therefore, they might not take into account considerable disparities in price between different, but comparably effective, options for patients. As a result, both patients and clinicians are often insulated from the direct financial impact of selecting a higher-priced product. Due to these dynamics, pharmaceutical marketing can significantly impact patient and clinician decisions that then greatly affect outcomes, in addition to draining government and health care Pharmaceutical companies spend billions system resources. on marketing $20.3B Marketing tactics can drive overprescribing through higher doses and longer courses of treatment than are necessary, as well as overuse $15.6B of newer, higher-priced drugs instead -

Pharmaceutical Sales Representatives
[Chapter 4, 28 April] Chapter 4 Pharmaceutical sales representatives Andy Gray, Jerome Hoffman and Peter R Mansfield The presence of pharmaceutical industry sales representatives almost seems a fact of life at many modern medical centres and universities around the world. Many medical and pharmacy students come into contact with pharmaceutical industry sales representatives during their training. Later on in the careers of many health professionals, encounters with sales representatives can occur on a daily basis, taking up a substantial portion of a busy health professional s time. However, health professionals have a choice in the matter - they may choose not to see pharmaceutical sales representatives at all or they may attempt to manage such interactions.’ This chapter aims to provide information to help you make up your own mind on this issue. This choice has important consequences for health professionals practice and patients, so requires careful consideration. ’ Aims of this chapter By the end of the session based on this chapter, you should be able to answer a series of questions on your interactions with sales representatives: In what ways, if any, might I hope to benefit from meeting with sales representatives? How are sales representatives selected, trained, supported and managed? What information do sales representatives provide? How might contact with sales representatives influence me in a positive or negative way? Should I have contact with sales representatives at all? Is it possible, if I choose to have contact with sales representatives, to minimise the potential harm and maximise the potential benefit for my professional development and practice? This chapter presents evidence that we believe can be helpful in addressing these questions, and ends with a series of activities that will allow students to work on the issue in more depth. -

Big Bang Health Care Reform — Does It Work?: the Case of Britain’S 1991 National Health Service Reforms
Big Bang Health Care Reform — Does It Work?: The Case of Britain’s 1991 National Health Service Reforms RUDOLF KLEIN University o f Bath, England ealth care reform has b e e n o n e of the worldwide epidemics of the 1990s. But among the many coun tries that have either attempted or contemplated the reform H of their health care systems (Hurst 1992; Organization for Economic Co-operation and Development 1994) Britain stands out from the rest. The reforms of the National Health Service (NHS) introduced in 1991 by Mrs. Thatcher’s Conservative government were driven by the much the same set of concerns and ideas that shaped the international vocabu lary of debate. In particular, they reflected the widely held belief that the best way of improving efficiency was to change the incentives to pro viders and that some form of marketlike competition was the best tool for achieving this aim (Saltman and van Otter 1992). In all these re spects, there was nothing all that special about Britain. What makes the British case special, and worthy of further study, however, is the ambi tious scope of the reforms and the relentless determination with which Mrs. Thatcher’s government implemented them. In the United States, the Clinton reforms plan foundered on the rocks of congressional oppo sition; in Sweden, a succession of local experiments and committees of inquiry failed to create a national consensus about the direction of health care policy; in the Netherlands, an ambitious plan of reform was The Milbank Quarterly, Vol. 73, No. 3, 1995 © 1995 Milbank Memorial Fund. -

Pharmaceutical Opioid Marketing and Physician Prescribing Behavior
Pharmaceutical Opioid Marketing and Physician Prescribing Behavior Svetlana N. Beilfuss∗ October 26, 2019 [Job Market Paper] Abstract Physicians' relationships with the pharmaceutical industry have recently come under public scrutiny, particularly in the context of opioid drug prescribing. This study examines the effect of doctor-industry marketing interactions on subsequent prescribing patterns of opioids using linked Medicare Part D and Open Payments data for the years 2014-2017. Results indicate that both the number and the dollar- value of marketing visits increase physicians' patented opioid claims. Furthermore, direct-to-physician marketing of safer abuse-deterrent formulations of opioids is the primary driver of positive and persistent spillovers on the prescribing of less safe generic opioids - a result that may be driven by insurance coverage policies. These findings suggest that pharmaceutical marketing efforts may have unintended public health implications. Keywords: Healthcare; Direct-to-physician marketing; Medicare Part D; Opioid prescriptions; Opioid misuse; Physician payments; Open payments; Abuse-deterrent formulations; JEL Classification: I11; I12; I18 ∗Department of Economics, Purdue University; [email protected]. Please check my website at www.svetlanabeilfuss.com for an up-to-date version of the draft. 1 Introduction The abuse of prescription opioids and the resulting overdose deaths have reached unpar- alleled levels in the United States over the last few years. In 2016, 63,632 individuals died from drug overdoses, with 66.4% of the cases involving opioids. Among opioid-related deaths, 40.4% involved prescription opioids (Centers for Disease Control and Prevention, 2018). Furthermore, two million people in the United States suffer from opioid addiction due to prescription opioid drugs (Schuchat et al., 2017). -

Nber Working Paper Series Pharmaceutical Use
NBER WORKING PAPER SERIES PHARMACEUTICAL USE FOLLOWING GENERIC ENTRY: PAYING LESS AND BUYING LESS Peter J. Huckfeldt Christopher R. Knittel Working Paper 17046 http://www.nber.org/papers/w17046 NATIONAL BUREAU OF ECONOMIC RESEARCH 1050 Massachusetts Avenue Cambridge, MA 02138 May 2011 This paper has benefited substantially from conversations with Hilary Hoynes, Doug Miller, Marianne Page, Ann Huff Stevens, and seminar participants at UC Davis and UC Berkeley. Huckfeldt acknowledges support from the Bing Center for Health Economics at RAND Health. All remaining errors are our own. The views expressed herein are those of the authors and do not necessarily reflect the views of the National Bureau of Economic Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- reviewed or been subject to the review by the NBER Board of Directors that accompanies official NBER publications. © 2011 by Peter J. Huckfeldt and Christopher R. Knittel. All rights reserved. Short sections of text, not to exceed two paragraphs, may be quoted without explicit permission provided that full credit, including © notice, is given to the source. Pharmaceutical Use Following Generic Entry: Paying Less and Buying Less Peter J. Huckfeldt and Christopher R. Knittel NBER Working Paper No. 17046 May 2011 JEL No. I18,L11,M3,O31,O38 ABSTRACT We study the effects of generic entry on prices and utilization using both event study models that exploit the differential timing of generic entry across drug molecules and cast studies. Our analysis examines drugs treating hypertension, high blood pressure, type 2 diabetes, and depression using price and utilization data from the Medical Expenditure Panel Survey. -

The Cost of Pushing Pills: a New Estimate of Pharmaceutical Promotion Expenditures in the United States Marc-André Gagnon*, Joel Lexchin
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by PubMed Central Policy Forum The Cost of Pushing Pills: A New Estimate of Pharmaceutical Promotion Expenditures in the United States Marc-André Gagnon*, Joel Lexchin n the late 1950s, the late and the critics’ portrayal of an The number of promotional meetings Democratic Senator Estes industry based on marketing-driven has increased dramatically in recent IKefauver, Chairman of the profiteering. years, going from 120,000 in 1998 to United States Senate’s Anti-Trust IMS, a firm specializing in 371,000 in 2004 [6]. In 2000, the top and Monopoly Subcommittee, put pharmaceutical market intelligence, ten pharmaceutical companies were together the first extensive indictment is usually considered to be the spending just under US$1.9 billion on against the business workings of the authority for assessing pharmaceutical 314,000 such events [7]. Third, IMS pharmaceutical industry. He laid three promotion expenditures. The US does not include the amount spent charges at the door of the industry: General Accounting Office, for on phase IV “seeding” trials, trials (1) Patents sustained predatory prices example, refers to IMS numbers in designed to promote the prescription and excessive margins; (2) Costs and concluding that “pharmaceutical of new drugs rather than to generate prices were extravagantly increased by companies spend more on research scientific data. In 2004, 13.2% (US$4.9 large expenditures in marketing; and and development initiatives than on billion) of R&D expenditures by (3) Most of the industry’s new products all drug promotional activities” [3]. -

Prevention Provisions in the Affordable Care Act Gail Shearer, MPP
American Public Health Association OCTOBER 2010 Prevention Provisions in the Affordable Care Act Gail Shearer, MPP Executive Summary n 2010 Congress enacted the Affordable Care Act, a historic and vigorously debated law designed to dramatically overhaul the health system. Included in the Affordable Care Act are comprehensive prevention provisions consistent with those called for by the American Public Health Association I (APHA) in its health reform agenda and supported by other leading experts in population health and prevention.12 The Affordable Care Act, if it is adequately funded, effectively implemented, and creatively leveraged through public and private-sector partnerships, will mark the turning point in the fundamental nature of our health system, initiating the transformation of our health system from one that treats sickness to one that promotes health and wellness. This issue brief begins (Section III) by summarizing the state of public health in the United States, including some measures of the growth of preventable diseases. Section IV describes the major provisions of the Affordable Care Act that address prevention through: (1) investing in public health; (2) educating the public; (3) expanding insurance coverage and requiring that health insur- ance include recommended preventive benefits; and (4) building capacity for better prevention in the future through demonstrations, research and evaluation. 800 I Street, NW • Washington, DC 20001-3710 • 202-777-APHA • fax: 202-777-2534 • www.apha.org Section V identifies key implementation