NOTA Notes on the Thinocorids of the High Andes of Bolivia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Phylogenetic Reanalysis of Strauch's Osteological Data Set for The
TheCondor97:174-196 0 The Cooper Ornithological Society 1995 PHYLOGENETIC REANALYSIS OF STRAUCH’S OSTEOLOGICAL DATA SET FOR THE CHARADRIIFORMES PHILIP c. CHU Department of Biology and Museum of Zoology The University of Michigan, Ann Arbor, MI 48109 Abstract. Strauch’s (1978) compatibility analysisof relationshipsamong the shorebirds (Charadriifonnes) was the first study to examine the full range of charadriifonn taxa in a reproducibleway. SubsequentlyMickevich and Parenti (1980) leveled seriouscharges against Strauch’s characters,method of phylogenetic inference, and results. To account for these charges,Strauch ’s characterswere re-examined and recoded, and parsimony analyseswere performed on the revised matrix. A parsimony analysison 74 taxa from the revised matrix yielded 855 shortesttrees, each length = 286 and consistencyindex = 0.385. In each shortest tree there were two major lineages,a lineageof sandpiper-likebirds and a lineageof plover- like birds; the two formed a monophyletic group, with the auks (Alcidae) being that group’s sister taxon. The shortest trees were then compared with other estimates of shorebird re- lationships, comparison suggestingthat the chargesagainst Strauch’s results may have re- sulted from the Mickevich and Parenti decisions to exclude much of Strauch’s character evidence. Key words: Charadrilformes; phylogeny; compatibility analysis: parsimony analysis; tax- onomic congruence. INTRODUCTION Strauch scored 227 charadriiform taxa for 70 The investigation of evolutionary relationships characters. Sixty-three of the characters were among shorebirds (Aves: Charadriiformes) has a taken from either the skull or postcranial skel- long history (reviewed in Sibley and Ahlquist eton; the remaining seven involved the respec- 1990). Almost all studies used morphology to tive origins of three neck muscles, as published make inferences about shared ancestry; infer- in Burton (1971, 1972, 1974) and Zusi (1962). -
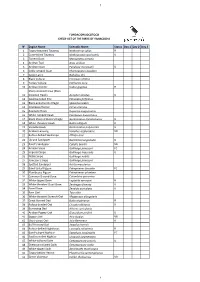
N° English Name Scientific Name Status Day 1
1 FUNDACIÓN JOCOTOCO CHECK-LIST OF THE BIRDS OF YANACOCHA N° English Name Scientific Name Status Day 1 Day 2 Day 3 1 Tawny-breasted Tinamou Nothocercus julius R 2 Curve-billed Tinamou Nothoprocta curvirostris U 3 Torrent Duck Merganetta armata 4 Andean Teal Anas andium 5 Andean Guan Penelope montagnii U 6 Sickle-winged Guan Chamaepetes goudotii 7 Cattle Egret Bubulcus ibis 8 Black Vulture Coragyps atratus 9 Turkey Vulture Cathartes aura 10 Andean Condor Vultur gryphus R Sharp-shinned Hawk (Plain- 11 breasted Hawk) Accipiter striatus U 12 Swallow-tailed Kite Elanoides forficatus 13 Black-and-chestnut Eagle Spizaetus isidori 14 Cinereous Harrier Circus cinereus 15 Roadside Hawk Rupornis magnirostris 16 White-rumped Hawk Parabuteo leucorrhous 17 Black-chested Buzzard-Eagle Geranoaetus melanoleucus U 18 White-throated Hawk Buteo albigula R 19 Variable Hawk Geranoaetus polyosoma U 20 Andean Lapwing Vanellus resplendens VR 21 Rufous-bellied Seedsnipe Attagis gayi 22 Upland Sandpiper Bartramia longicauda R 23 Baird's Sandpiper Calidris bairdii VR 24 Andean Snipe Gallinago jamesoni FC 25 Imperial Snipe Gallinago imperialis U 26 Noble Snipe Gallinago nobilis 27 Jameson's Snipe Gallinago jamesoni 28 Spotted Sandpiper Actitis macularius 29 Band-tailed Pigeon Patagoienas fasciata FC 30 Plumbeous Pigeon Patagioenas plumbea 31 Common Ground-Dove Columbina passerina 32 White-tipped Dove Leptotila verreauxi R 33 White-throated Quail-Dove Zentrygon frenata U 34 Eared Dove Zenaida auriculata U 35 Barn Owl Tyto alba 36 White-throated Screech-Owl Megascops -

Avian Nesting and Roosting on Glaciers at High Elevation, Cordillera Vilcanota, Peru
The Wilson Journal of Ornithology 130(4):940–957, 2018 Avian nesting and roosting on glaciers at high elevation, Cordillera Vilcanota, Peru Spencer P. Hardy,1,4* Douglas R. Hardy,2 and Koky Castaneda˜ Gil3 ABSTRACT—Other than penguins, only one bird species—the White-winged Diuca Finch (Idiopsar speculifera)—is known to nest directly on ice. Here we provide new details on this unique behavior, as well as the first description of a White- fronted Ground-Tyrant (Muscisaxicola albifrons) nest, from the Quelccaya Ice Cap, in the Cordillera Vilcanota of Peru. Since 2005, .50 old White-winged Diuca Finch nests have been found. The first 2 active nests were found in April 2014; 9 were found in April 2016, 1 of which was filmed for 10 d during the 2016 nestling period. Video of the nest revealed infrequent feedings (.1 h between visits), slow nestling development (estimated 20–30 d), and feeding via regurgitation. The first and only active White-fronted Ground-Tyrant nest was found in October 2014, beneath the glacier in the same area. Three other unoccupied White-fronted Ground-Tyrant nests and an eggshell have been found since, all on glacier ice. At Quelccaya, we also observed multiple species roosting in crevasses or voids (caves) beneath the glacier, at elevations between 5,200 m and 5,500 m, including both White-winged Diuca Finch and White-fronted Ground-Tyrant, as well as Plumbeous Sierra Finch (Phrygilus unicolor), Rufous-bellied Seedsnipe (Attagis gayi), and Gray-breasted Seedsnipe (Thinocorus orbignyianus). These nesting and roosting behaviors are all likely adaptations to the harsh environment, as the glacier provides a microclimate protected from precipitation, wind, daily mean temperatures below freezing, and strong solar irradiance (including UV-B and UV-A). -

Argentina & Chile
Argentina & Chile Southern Patagonia & Torres del Paine 3rd December to 13th December 2022 (11 days) Magellanic Plover by Rich Lindie Our tour through Argentina’s Southern Patagonia takes us on an amazing adventure through the southern portion of this incredible continent in search of the unique Magellanic Plover at Laguna Nímez, the rare White-bellied Seedsnipe on the wide-open grassy plains of northern Tierra del Fuego, and the charismatic Magellanic Woodpecker. Our journey begins in Los Glaciares National Park, famous in birding circles for its population of the impressive Andean Condors, uncommon Bronze-winged Duck, Chilean Flicker and the strange Rufous-tailed Plantcutter. Crossing the border into Chile, we spend a few days at possibly the most scenically impressive site on a tour of grand vistas - Torres del Paine. Aside from being a staggeringly spectacular stretch of mountains - quite possibly the most attractive scenery of all the Andes - Torres del Paine National Park also happens to be one of the best places in the world to see the mighty Puma. Next, we travel to the Punta Arenas, exploring the most southern continental locations for RBL Argentina - Southern Patagonia Itinerary 2 their plethora of rare and endemic species, both by road and ferry. As the tour draws to a close, we will be searching for birds in the dramatic and fabled landscapes of Tierra del Fuego. Here a visit to Tierra del Fuego National Park could produce Austral Pygmy Owl, White-throated Treerunner and the fantastic Magellanic Woodpecker. In the Beagle Channel, we hope for Magellanic Diving Petrel, Fuegian Steamer Duck and White-throated Caracara, while in the Rio Grande area we search for the rare White-bellied Seedsnipe. -

Bolivia: Endemic Macaws & More!
BOLIVIA: ENDEMIC MACAWS & MORE! PART II: FOOTHILLS, CLOUDFORESTS & THE ALTIPLANO SEPTEMBER 28–OCTOBER 8, 2018 Male Versicolored Barbet – Photo Andrew Whittaker LEADERS: ANDREW WHITTAKER & JULIAN VIDOZ LIST COMPILED BY: ANDREW WHITTAKER VICTOR EMANUEL NATURE TOURS, INC. 2525 WALLINGWOOD DRIVE, SUITE 1003 AUSTIN, TEXAS 78746 WWW.VENTBIRD.COM Bolivia continued to exceed expectations on Part 2 of our tour! Steadily climbing up into the mighty ceiling of South America that is the Andes, we enjoyed exploring many more new, different, and exciting unspoiled bird-rich habitats, including magical Yungas cloudforest stretching as far as the eye could see; dry and humid Puna; towering snow-capped Andean peaks; vast stretches of Altiplano with its magical brackish lakes filled with immense numbers of glimmering flamingoes, and one of my favorite spots, the magnificent and famous Lake Titicaca (with its own flightless grebe). An overdose of stunning Andean scenery combined with marvelous shows of flowering plants enhanced our explorations of a never-ending array of different and exciting microhabitats for so many special and interesting Andean birds. We were rewarded with a fabulous trip record total of 341 bird species! Combining our two exciting Bolivia tours (Parts 1 and 2) gave us an all-time VENT record, an incredible grand total of 656 different bird species and 15 mammals! A wondrous mirage of glimmering pink hues of all three species of flamingos on the picturesque Bolivian Altiplano – Photo Andrew Whittaker Stunning Andes of Bolivia near Soroto on a clear day of our 2016 trip – Photo Andrew Whittaker Victor Emanuel Nature Tours 2 Bolivia Part 2, 2018 We began this second part of our Bolivian bird bonanza in the bustling city of Cochabamba, spending a fantastic afternoon birding the city’s rich lakeside in lovely late afternoon sun. -

Transport of Water by Adult Sandgrouse to Their Young Tom J
THE CONDOR VOLUME69 JULY-AUGUST,1967 NUMBER4 TRANSPORT OF WATER BY ADULT SANDGROUSE TO THEIR YOUNG TOM J. CADE and GORDONL. MACLEAN In 1896 the English aviculturist Meade-Waldo published an astonishing and seemingly incredible account of how the males of sandgrouse that he successfully bred in captivity carried water to their young in their breast feathers. To quote from his original report: As soon as the young were out of the nest (when twelve hours old) a very curious habit developed itself in the male. He would rub his breast violently up and down on the ground, a motion quite distinct from dusting, and when all awry he would get into his drinking water and saturate the feathers of the under parts. When soaked he would go through the motions of flying away, nodding his head, etc. Then, remembering his family were close by, would run up to the hen, make a demonstration, when the young would run out, get under him, and suckthe water from his breast. This is no doubt the way that water is conveyed to the young when far out on waterless plains. The young . are very independent, eating hard seed and weeds from the first, and roosting independently of their parents at ten days old (Meade-Waldo, 1896). See also Meade- Waldo (1921). Despite the fact that .Meade-Waldo (1897 ; 1921) observed 61 broods from three different species of sandgrouse hatched in his aviaries between 189.5 and l915, and soon received confirmation from another breeder for two species (St. Quintin, 1905), and despite the fact that field naturalists and native hunters have frequently observed wild male sandgrouse wetting their breast feathers at water holes in the way described (Meade-Waldo, 1906; Buxton, 1923; Heim de Balsac, 1936; Hoesch, 1955), the idea that the young do receive water in this exceptional way has met with a great deal of scepticism (Archer and Godman, 1937; Meinertzhagen, 1954, 1964; Hiie and Etchkcopar, 1957; Schmidt-Nielsen, 1964). -

Native Grasslands and the Plains- Wanderer
Birds Australia Conservation Statement No. 1 NATIVE GRASSLANDS AND THE PLAINS- WANDERER by David Baker-Gabb SUMMARY: Lowland native grasslands are among the most depleted ecosystems in south-eastern Australia, and contain a dispro- portionately large number of threatened plant species. Threatened grassland fauna such as the Plains-wanderer have suffered a similar decline. Plains-wanderers are permanent residents in their favoured patches of sparse native grassland. However, they cannot survive where these grasslands are converted to crops or dense introduced pasture, or are overgrazed by stock. In the five years since the publication of the first RAOU Plains-wanderer Conser- vation Statement in 1993, considerable effort has been expended in surveying and studying the temperate native grasslands of South Australia, Victoria and New South Wales. This work has revealed that the status of the Plains-wanderer is worse than previously thought. A very significant new threat has emerged from an expanding rice industry in the Plains-wanderer’s stronghold, the Riverina of New South Wales. The situation for Plains- wanderers in Queensland remains unclear, but there is no reason to suspect that it is improving given the expansion of the cotton industry and conversion of native grasslands to introduced pasture there. On the positive side, recent studies have provided refined information on managing native grasslands to maintain their biodiversity, and they provide some clear targets for urgent conservation action. High quality native grassland with sparse open structure, NSW Riverina – typically favoured Plains- wanderer habitat. Photo by Marianne Porteners/ Royal Botanic Gardens Sydney Female Plains-wanderer. Photo by Tom Wheller Supplement to Wingspan, vol. -

Chile Trip Report April 2015
BIRDING CHILE APRIL 11 – 29, 2015 A BIRDING AND LOGISTICS REPORT We visited Chile at a rather unconventional time, as most birding groups visit the country in the austral spring/summer. This report was mostly written at the time of the trip, but due to an additional 4 months of traveling through the tropics it never was finished. Although this report doesn’t include the depth and breadth of information I originally planned it to have, I decided to publish it anyway. There is very little information available for birding trips to Chile in April, so hopefully this will be helpful to others that decide to travel to the country during the austral fall. For blog posts on the trip (and a lot more pictures) visit the Chile section of Budgetbirders.com TRIP ITINERARY April 11 – Arrived Santiago 0300, SUMMARY departed for Punta Arenas 0800 WHEN and arrived 1630 Most birding groups visit Chile during the austral spring or April 12 – Laguna Los Palos, summer (Nov-Mar) when resident birds are breeding and Route 9, Puerto Natales, Torres migrants are present. Due to schedule constraints we visited Del Paine Chile in the austral fall. Despite not being the prime time of April 13 – Torres Del Paine (Lago year, overall we had a very successful trip. Most of the typical Gray Trail), Sierra Bagueles Chilean target species were still present but we missed April 14 – Route 405, Port several austral migrants, most notably 3 species from Delgada Ferry, Porvenir tyrannidae, White-sided Hillstar, Austral Rail, and Creamy- rumped Miner. April 15 – Laguana Verde, Parque Penguinos Rey TOTAL # OF SPECIES: April 16 – Porvenir, seawatch, Birding highlights included seeing a total of 241 species of ferry to Puenta Arenas which 10 were Chilean endemics. -

A First Documented Brazilian Record of Least Seedsnipe Thinocorus Rumicivorus Eschscholtz, 1829 (Thinocoridae)
Revista Brasileira de Ornitologia, 20(4), 455-457 Nota/SHort-CommUNIcatIon Dezembro de 2012 / December 2012 A first documented Brazilian record of Least Seedsnipe Thinocorus rumicivorus Eschscholtz, 1829 (Thinocoridae) Felipe Castro1, João Castro1, Aluisio Ramos Ferreira2, Marco Aurélio Crozariol3,6 and Alexander Charles Lees4,5 1 Rua Tainha, 345, Bairro Sítio Ressaca, Ubatuba, SP. CEP: 11680-000. Brazil. 2 Rua Joaquim do Prado, 413, Cruzeiro, SP. CEP: 12701-370. Brazil. 3 Clube de Observadores de Aves do Vale do Paraíba Paulista – COAVAP; Programa de Pós-Graduação em Zoologia. Museu Nacional/UFRJ, Departamento de Vertebrados, Setor de Ornitologia, Quinta da Boa Vista, São Cristóvão, Rio de Janeiro, RJ. CEP: 20940-040. Brazil. 4 Coordenação de Zoologia, Museu Paraense Emílio Goeldi, CP 399, Belém, Pará, Brazil. 5 Department of Zoology, University of Cambridge, Cambridge CB2 3EJ, UK. 6 Corresponding author: [email protected] Received on 25 July 2012. Accepted on 23 August 2012. ABSTRACT: Herein we present the first documented record of the Least Seedsnipe Thinocorus rumicivorus (Eschscholtz, 1829) for Brazil. On the 21 April 2012 a juvenile T. rumicivorus was photographed and sound-recorded by birdwatchers on the beach at Ubatumirim in the municipality of Ubatuba, on the northern São Paulo state coast. This is the first documented record of any seedsnipe (Thinocoridae) for Brazil. Its behaviour and the circumstances and potential drivers of its vagration are discussed. KEY-WORDS: birdwatching; Eragrostis; Thinocorus; vagrancy. At mid-morning on 21 April 2012, F. C., J. C. and Figure 1a). The bird only flew on rare occasions when A. R. F. were birdwatching on the beach at Ubatumirim totally encircled by the watching observers or when (23°19’53.53”S; 44°54’34.89”W) in the municipality of approached rapidly by locals on foot or on bicycles. -

Limosa Haemastica (Linnaeus, 1758): First Record from South Istributio
ISSN 1809-127X (online edition) © 2010 Check List and Authors Chec List Open Access | Freely available at www.checklist.org.br Journal of species lists and distribution N Aves, Charadriiformes, Scolopacidae, Limosa haemastica (Linnaeus, 1758): First record from South ISTRIBUTIO D Shetland Islands and Antarctic Peninsula, Antarctica 1,2* 1 1 1, 2 3 RAPHIC Mariana A. Juáres , Marcela M. Libertelli , M. Mercedes Santos , Javier Negrete , Martín Gray , G 1 1,2 4 1 1 EO Matías Baviera , M. Eugenia Moreira , Giovanna Donini , Alejandro Carlini and Néstor R. Coria G N O 1 Instituto Antártico Argentino, Departmento Biología, Aves, Cerrito 1248, C1010AAZ. Buenos Aires, Argentina. OTES 3 Administración de Parques Nacionales (APN). Avenida Santa Fe 690, C1059ABN. Buenos Aires, Argentina. N 4 2 JarConsejodín Zoológico Nacional de de Buenos Investigaciones Aires. República Científicas de lay TécnicasIndia 2900, (CONICET). C1425FCF. Rivadavia Buenos Aires,1917, Argentina.C1033AAJ. Buenos Aires, Argentina. * Corresponding author. E-mail: [email protected] Abstract: We report herein the southernmost record of the Hudsonian Godwit (Limosa haemastica), at two localities in the Antarctic: Esperanza/Hope Bay (January 2005) and 25 de Mayo/King George Island (October 2008). On both occasions a pair of specimens with winter plumage was observed. The Hudsonian Godwit Limosa haemastica (Linnaeus tide and each time birds were feeding in the intertidal 1758) is a neartic migratory species that breeds in Alaska zone. These individuals showed the winter plumage and Canada during summer and spends its non-breeding pattern: dark reddish chest and white ventral region, black period in the southernmost regions of South America primaries and tail feathers, a long upturned bill pink at during the boreal winter. -
The Wandererthe Wanderer
The WandererThe Wanderer This occasional newsletter covers news, and information about Plains-wanderer Winter 2016 Volume 1 Issue 2 conservation and management. Past newsletters are available via a link on the Trust for Nature website http://www.trustfornature.org.au/our-conservation-work/priority-species/plains- Inside this issue: wanderer/ and on Facebook: https://www.facebook.com/TrustforNatureVictoria Thanks to all of the contributors to this issue, and particularly for the review of Queensland records by Plains-wanderer 1 Maree Rich and colleagues. If you have articles that may be of interest to others, please send copies to the Recovery Plan adopted editor David Baker-Gabb at [email protected] Plains-wanderer on 1 NSW Iconic Species Plains-wanderer Recovery Plan The Birds and the Peas list adopted Grasslands Field Day Grasslands Field Day 1 On 28 June 2016 the National Recovery Plan Join the Northern Plains Conservation Plains-wanderers in 2 for the Plains-wanderer came into effect under Management Network, Trust for Nature Qld; a summary of the the EPBC Act. The final recovery plan can and Dr David Baker-Gabb in launching last 30 years be accessed at: the Managing Native Grasslands for Plains- Plains-wanderer 6 http://www.environment.gov.au/biodivers wanderers Field Guide. monitoring results for ity/threatened/recovery-plans/plains- Hear from Dr Dan Harley about the Zoos Victoria 2015-2016 wanderer-2016 Victoria Fighting Extinction programme Plains-wanderer 7 and their plan to start captive-breeding monitoring and surveys Plains-wanderers in 2017. Plains-wanderer on NSW Iconic from NSW 2014-16 Walk with grassland ecologist Paul Species list Boolcoomatta Reserve, 8 Foreman and learn how to survey for South Australia threatened grassland plants. -

SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does Not Include Alcidae
SHOREBIRDS (Charadriiformes*) CARE MANUAL *Does not include Alcidae CREATED BY AZA CHARADRIIFORMES TAXON ADVISORY GROUP IN ASSOCIATION WITH AZA ANIMAL WELFARE COMMITTEE Shorebirds (Charadriiformes) Care Manual Shorebirds (Charadriiformes) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Charadriiformes Taxon Advisory Group. (2014). Shorebirds (Charadriiformes) Care Manual. Silver Spring, MD: Association of Zoos and Aquariums. Original Completion Date: October 2013 Authors and Significant Contributors: Aimee Greenebaum: AZA Charadriiformes TAG Vice Chair, Monterey Bay Aquarium, USA Alex Waier: Milwaukee County Zoo, USA Carol Hendrickson: Birmingham Zoo, USA Cindy Pinger: AZA Charadriiformes TAG Chair, Birmingham Zoo, USA CJ McCarty: Oregon Coast Aquarium, USA Heidi Cline: Alaska SeaLife Center, USA Jamie Ries: Central Park Zoo, USA Joe Barkowski: Sedgwick County Zoo, USA Kim Wanders: Monterey Bay Aquarium, USA Mary Carlson: Charadriiformes Program Advisor, Seattle Aquarium, USA Sara Perry: Seattle Aquarium, USA Sara Crook-Martin: Buttonwood Park Zoo, USA Shana R. Lavin, Ph.D.,Wildlife Nutrition Fellow University of Florida, Dept. of Animal Sciences , Walt Disney World Animal Programs Dr. Stephanie McCain: AZA Charadriiformes TAG Veterinarian Advisor, DVM, Birmingham Zoo, USA Phil King: Assiniboine Park Zoo, Canada Reviewers: Dr. Mike Murray (Monterey Bay Aquarium, USA) John C. Anderson (Seattle Aquarium volunteer) Kristina Neuman (Point Blue Conservation Science) Sarah Saunders (Conservation Biology Graduate Program,University of Minnesota) AZA Staff Editors: Maya Seaman, MS, Animal Care Manual Editing Consultant Candice Dorsey, PhD, Director of Animal Programs Debborah Luke, PhD, Vice President, Conservation & Science Cover Photo Credits: Jeff Pribble Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management.