The Persistence of Common Wombats in Road Impacted Environments
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

NSW Light Vehicles Agricultural and Load Exemption Order 2019
NSW Light Vehicle Agricultural and Load Exemption Order 2019 Notice of suitable routes and areas Travel Times, Zones and Travel Conditions – Load Carrying vehicles In accordance with the Order, this notice identifies routes and zones that Roads and Maritime Services has identified as suitable for use at the times and in the manner specified for each route or zone. Part 1 – NSW Urban Zone For the purposes of this Part the NSW Urban Zone is defined as the area bounded by and including: • the Pacific Ocean and the North Channel of the Hunter River, then • north from Stockton bridge along Nelson Bay Road (MR108) to Williamtown, then • west along Cabbage Tree Road (MR302) to Masonite Road near Tomago, then • along Masonite Road to the Pacific Highway (HW10) at Heatherbrae, then • south along the Pacific Highway (HW10) to Hexham, then • west along the New England Highway (HW9) to Weakleys Drive Thornton, then • south along Weakleys Drive to the F3 Sydney Newcastle Freeway at Beresfield, then • along the F3 Sydney Newcastle Freeway to the Hawkesbury River bridge, then • along the Hawkesbury River and the Nepean River to Cobbity, then • a line drawn south from Cobbitty to Picton, then • via Picton Road and Mount Ousley Road (MR95) to the start of the F6 Southern Freeway at Mount Ousley, then • via the F6 Southern Freeway to the Princes Highway at West Wollongong, then • the Princes Highway and Illawarra Highway to Albion Park with a branch west on West Dapto Road to Tubemakers, then • Tongarra Road to the Princes Highway, then • Princes Highway south to the intersection of South Kiama Drive at Kiama Heights, then • a straight line east to the Pacific Ocean. -

Speed Camera Locations
April 2014 Current Speed Camera Locations Fixed Speed Camera Locations Suburb/Town Road Comment Alstonville Bruxner Highway, between Gap Road and Teven Road Major road works undertaken at site Camera Removed (Alstonville Bypass) Angledale Princes Highway, between Hergenhans Lane and Stony Creek Road safety works proposed. See Camera Removed RMS website for details. Auburn Parramatta Road, between Harbord Street and Duck Street Banora Point Pacific Highway, between Laura Street and Darlington Drive Major road works undertaken at site Camera Removed (Pacific Highway Upgrade) Bar Point F3 Freeway, between Jolls Bridge and Mt White Exit Ramp Bardwell Park / Arncliffe M5 Tunnel, between Bexley Road and Marsh Street Ben Lomond New England Highway, between Ross Road and Ben Lomond Road Berkshire Park Richmond Road, between Llandilo Road and Sanctuary Drive Berry Princes Highway, between Kangaroo Valley Road and Victoria Street Bexley North Bexley Road, between Kingsland Road North and Miller Avenue Blandford New England Highway, between Hayles Street and Mills Street Bomaderry Bolong Road, between Beinda Street and Coomea Street Bonnyrigg Elizabeth Drive, between Brown Road and Humphries Road Bonville Pacific Highway, between Bonville Creek and Bonville Station Road Brogo Princes Highway, between Pioneer Close and Brogo River Broughton Princes Highway, between Austral Park Road and Gembrook Road safety works proposed. See Auditor-General Deactivated Lane RMS website for details. Bulli Princes Highway, between Grevillea Park Road and Black Diamond Place Bundagen Pacific Highway, between Pine Creek and Perrys Road Major road works undertaken at site Camera Removed (Pacific Highway Upgrade) Burringbar Tweed Valley Way, between Blakeneys Road and Cooradilla Road Burwood Hume Highway, between Willee Street and Emu Street Road safety works proposed. -

Snowy Mountains Region Visitors Guide
Snowy Mountains Region Visitors Guide snowymountains.com.au welcome to our year-round The Snowy Mountains is the ultimate adventure four-season holiday destination. There is something very special We welcome you to come and see about the Snowy Mountains. for yourself. It will be an escape that you will never forget! playground It’s one of Australia’s only true year- round destinations. You can enjoy Scan for more things to do the magical winter months, when in the Snowy Mountains or visit snowymountains.com.au/ a snow experience can be thrilling, things-to-do adventurous and relaxing all at Contents the same time. Or see this diverse Kosciuszko National Park ............. 4 region come alive during the Australian Folklore ........................ 5 spring, summer and autumn Snowy Hydro ............................... 6 months with all its wonderful Lakes & Waterways ...................... 7 activities and attractions. Take a Ride & Throw a Line .......... 8 The Snowy Mountains is a natural Our Communities & Bombala ....... 9 wonder of vast peaks, pristine lakes and rushing rivers and streams full of Cooma & Surrounds .................. 10 life and adventure, weaving through Jindabyne & Surrounds .............. 11 unique and interesting landscapes. Tumbarumba & Surrounds ......... 12 Take your time and tour around Tumut & Surrounds .................... 13 our iconic region enjoying fine Our Alpine Resorts ..................... 14 food, wine, local produce and Go For a Drive ............................ 16 much more. Regional Map ............................. 17 Regional Events & Canberra ...... 18 “The Snowy Mountains Getting Here............................... 19 – there’s more to it Call Click Connect Visit .............. 20 than you think!” 2 | snowymountains.com.au snowymountains.com.au | 3 Australian folklore Horse riding is a ‘must do’, when and friends. -

Capital Coast and Country Touring Route Canberra–Tablelands–Southern Highlands– Snowy Mountains–South Coast
CAPITAL COAST AND COUNTRY TOURING ROUTE CANBERRA–TABLELANDS–SOUTHERN HIGHLANDS– SNOWY MOUNTAINS–SOUTH COAST VISITCANBERRA CAPITAL COAST AND COUNTRY TOURING 1 CAPITAL, COAST AND COUNTRY TOURING ROUTE LEGEND Taste the Tablelands SYDNEY Experience the Southern Highlands SYDNEY AIRPORT Explore Australia’s Highest Peak Enjoy Beautiful Coastlines Discover Sapphire Waters and Canberra’s Nature Coast Royal Southern Highlands National Park Young PRINCES HWY (M1) Mittagong Wollongong LACHLAN Boorowa VALLEY WAY (B81) Bowral ILL AWARR Harden A HWY Shellharbour Fitzroy Robertson HUME HWY (M31) Falls Kiama Goulburn Kangaroo Yass Gerringong Valley HUME HWY (M31) Jugiong Morton Collector National Nowra Shoalhaven Heads Murrumbateman FEDERAL HWY (M23) Park Seven Mile Beach BARTON HWY (A25) Gundaroo National Park Gundagai Lake Jervis Bay SNOWY MOUNTAINS HWY (B72) Hall George National Park Brindabella National Bungendore Sanctuary Point Park Canberra KINGS HWY (B52) Jervis Bay International Morton Conjola Sussex CANBERRA Airport National National Inlet Park Park TASMAN SEA Tumut Queanbeyan Lake Conjola Tidbinbilla Budawang Braidwood National Mollymook Park Ulladulla PRINCES HWY (A1) Namadgi (B23) HWY MONARO Murramarang Yarrangobilly National Park National Park Batemans Bay AUSTRALIA Yarrangobilly Mogo Caves Bredbo CANBERRA SYDNEY PRINCES HWYMoruya (A1) MELBOURNE Bodalla Tuross Head Snowy Mountains Cooma SNOWY MOUNTAINS HWY (B72) Narooma KOSCIUZSKO RD Eurobadalla Montague Perisher National Park Tilba Island Jindabyne Thredbo Wadbilliga Bermagui Alpine National -

L'etape-ROAD CLOSURE NOTIFICATION
L’Étape Australia by le Tour de France is back for its second edition, taking place on 2 December 2017 in the NSW Snowy Mountains. L’Étape Australia by Le Tour de France is the only cycling event run under professional conditions for amateurs, with closed roads, a challenging route and with a Sprint and two King of the Mountain sections. This world class event will provide a platform to showcase the spectacular Snowy Mountains region during the summer months, generating a real boost to the local economy. In the week leading up to the event day, crews will be busy setting up event areas around the course route. During these activities, all roads will remain open with local traffic control installed to safely guide pedestrians and vehicles around the work areas as required. The table below lists all road closure times across the course on 2 December 2017. For further details relating to the Road Closures, please visit www.livetraffic.com or phone 132 701. Alpine Way between Thredbo & Kosciuszko Road 5:30am 8:30am Kosciuszko Road between Alpine Way and Eucumbene 5:15am 9:15am Road Access to Jindabyne from Berridale via Dalgety Detour between 5:00am to 7:00am Eucumbene Road between Kosciuszko Road and Rocky 6:00am 10:50am Plains Road Rocky Plains Road between Eucumbene Road and 6:00am 10:50am Middlingbank Road Middlingbank Road between Rocky Plain Road and 6:00am 10:50am Kosciuszko Road Jindabyne Road between Middlingbank Road and Myack 6:00am 10:50am Street Myack Street between Kosciuszko Road and Dalgety Road 6:00am 12:45pm Dalgety Road between Myack Street and The Snowy River 7:15am 12:15pm Way Campbell Street through Dalgety 7:15am 12:15pm The Snowy River Way between Dalgety and Barry Way 7:15am 2:00pm Barry Way between The Snowy River Way and Kosciuszko 7:30am 2:00pm Road Kosciuszko Road between Alpine Way and Perisher 8:30am 4:30pm NOTE: Emergency Services will be available to access properties impacted by the event closures at all times in case of an emergency. -

Kosciuszko National Park Fire Management Strategydownload
Kosciuszko National Park Fire Management Strategy 2008–2013 Acknowledgment The Kosciuszko National Park Fire Management Strategy 2008 - 2013 recognises that the Park is within landscape that gives identity to Aboriginal people, who have traditional and historical connections to this land. Aboriginal people are recognised and respected as the original custodians of the lands, waters, animals and plants now within the Park. Their living and spiritual connections with the land through traditional laws, customs and beliefs passed on from their ancestors are also recognised. NPWS will continue to be committed to actively engage traditional custodians and relevant Aboriginal organisations, in protecting managing and interpreting the needs of Kosciuszko National Park. The landscapes of Kosciuszko National Park are the sum total of the interactions between the basic natural elements of water, air, rocks, soils, fire, plants and animals. Each of these living and non-living attributes have been altered by thousands of years of human habitation and use which have left behind layers of human artefacts, memories, stories and meanings. The management of these attributes, and the values bestowed upon them, is a complex task and will not always be based upon a complete understanding of the implications of individual management decisions. A precautionary and adaptive approach to management is required - one that appreciates the interconnective and inseparable nature of the elements of the landscape. (NPWS, 2006c pg 37) Kosciusko National Park Fire Management Strategy 2008 - 2013 ACKNOWLEDGMENTS This Fire Management Strategy was prepared by Jamie Molloy with the assistance of staff from the Snowy Mountains and South West Slopes Regions of the National Parks and Wildlife Service, staff from the Threatened Species Unit, the Reserve Conservation Planning & Performance Unit, and the Public Affairs Division of the Department of Environment and Climate Change. -

Planning Statement
Our Ref: DOC20/266610-3 Your Ref: Draft LSPS – e-mail dated 2 April 2020 General Manager Byron Shire Council PO Box 219 Mullumbimby NSW 2482 Attention: Ms Alex Caras Dear Mr Arnold RE: Byron Shire Council – Draft Local Strategic Planning Statement I refer to the e-mail from Mr Peter Cameron from the NSW Department of Planning, Industry and Environment dated 2 April 2020 about the Byron Shire Council’s Draft Local Strategic Planning Statement (Draft LSPS), seeking comments from the Department’s Biodiversity and Conservation Division (BCD) in the Environment, Energy and Science Group. I appreciate the opportunity to provide input. The BCD was formerly part of the Office of Environment and Heritage, but now forms part of a Group that has responsibilities relating to biodiversity (including threatened species and ecological communities, or their habitats), Aboriginal cultural heritage, National Parks and Wildlife Service estate, climate change, sustainability, flooding, coastal and estuary matters. We have undertaken a comprehensive review of the Draft LSPS and its associated strategies and plans. While we recognise there are restrictions on the length of LSPSs, generally, the Draft LSPS lacks specific actions for the Byron Shire Council (BSC) to satisfy the Directions of the North Coast Regional Plan (NCRP). In addition, the LSPS may be unable to achieve the desired themes and planning priorities, based on the planning priorities and actions contained therein. These issues are discussed in detail in Attachment 1 to this letter. In summary, the BCD recommends that: 1. The additional actions set out in Attachment 1 of this letter should be included within the Draft LSPS to ensure that biodiversity values are identified and protected. -
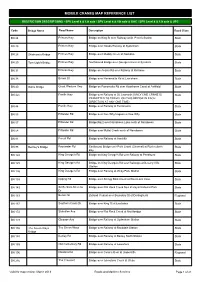
Mobile Crane Network
MOBILE CRANES MAP REFERENCE LIST RESTRICTION DESCRIPTIONS - SPV Level 4 & 12t axle / SPV Level 4 & 12t axle & UAC / SPV Level 4 & 12t axle & UPC Code Bridge Name Road Name Description Road Class BN 24 Princes Hwy Bridge on King St over Railway at St. Peter's Station State BN 25 Princes Hwy Bridge over Goods Railway at Sydenham State BN 28 Skidmore's Bridge Princes Hwy Bridge over Muddy Creek at Rockdale State BN 29 Tom Ugly's Bridge Princes Hwy Northbound Bridge over George's River at Sylvania State BN 31 Princes Hwy Bridge on Acacia Rd over Railway at Kirrawee State BN 38 Brown St Bridge over Parramatta Rd at Lewisham State BN 40 Battle Bridge Great Western Hwy Bridge on Parramatta Rd over Hawthorne Canal at Ashfield State BN 42c Pacific Hwy Bridge over Railway at St. Leonards (ONLY ONE CRANE IS State PERMITTED TO TRAVEL ON THIS BRIDGE IN EACH DIRECTION AT ANY ONE TIME) BN 44 Pacific Hwy Bridge over Railway at Turramurra State BN 55 Pittwater Rd Bridge over Dee Why Lagoon at Dee Why State BN 57 Pittwater Rd Bridge(No2) over Narrabeen Lake north of Narrabeen State BN 58 Pittwater Rd Bridge over Mullet Creek north of Narrabeen State BN 81 Forest Rd Bridge over Railway at Arncliffe State BN 98 Bentley's Bridge Bayswater Rd Eastbound Bridge over Park Creek (Channel) at Rushcutter's State Bay BN 124 King George's Rd Bridge on King George's Rd over Railway at Penshurst State BN 125 King George's Rd Bridge on King George's Rd over Railways at Beverly Hills State Station BN 126 King George's Rd Bridge over Railway at Wiley Park Station State -

Winter Wanderings
Winter wanderings Volume No 6, August 2014 2 | BWA August 2014 Bushwalk.com Magazine An electronic magazine for http://bushwalk.com Volume 6 August 2014 “We would like to start by acknowledging the Traditional Letter from Owners of this vast land on which we explore. We would 4 the Editor like to pay my respects to their Elders, past and present and thank them for their stewardship of this great south land.” Cover picture Dehydrating Food 6 Getting Started Hikers Helping Each Other up a Snowshoe walk Mountain by Rawpixel 12 Wheatley Circuit Editor Matt McClelland [email protected] Desig n manager Eva Gomišček Dunphy’s Kowmung Adventure [email protected] 16 Colong Wilderness Walk Sub-editor Stephen Lake Melbourne Please send any articles, suggestions or advertising enquires to the editor. 19 Sydney Harbour & Coast Walk Warning The Inaugural Foreshore Walk Like all outdoor pursuits, the activities described in this publication are dangerous. Undertaking them may result in loss, serious injury or death. The information in this publi- cation is without any warranty on accuracy or completeness. There may be significant omis- sions and errors. People who are interested in walking in the But Avalanches don’t areas concerned should make their own enquiries, and not rely 20 happen in Australia? fully on the information in this publication. Thoughts on Alpine safety The publisher, editor, authors or any other entity or person will not be held responsible for any loss, injury, claim or liability of any kind resulting from people using information in this publi- cation. Photo gallery Please consider joining a 24 Winter beauty walking club or undertaking formal training in other ways to ensure you are well prepared for any activities you are planning. -
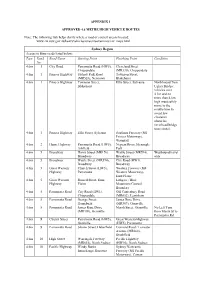
APPENDIX 1 APPROVED 4.6 METRE HIGH VEHICLE ROUTES Note: The
APPENDIX 1 APPROVED 4.6 METRE HIGH VEHICLE ROUTES Note: The following link helps clarify where a road or council area is located: www.rta.nsw.gov.au/heavyvehicles/oversizeovermass/rav_maps.html Sydney Region Access to State roads listed below: Type Road Road Name Starting Point Finishing Point Condition No 4.6m 1 City Road Parramatta Road (HW5), Cleveland Street Chippendale (MR330), Chippendale 4.6m 1 Princes Highway Sydney Park Road Townson Street, (MR528), Newtown Blakehurst 4.6m 1 Princes Highway Townson Street, Ellis Street, Sylvania Northbound Tom Blakehurst Ugly's Bridge: vehicles over 4.3m and no more than 4.6m high must safely move to the middle lane to avoid low clearance obstacles (overhead bridge truss struts). 4.6m 1 Princes Highway Ellis Street, Sylvania Southern Freeway (M1 Princes Motorway), Waterfall 4.6m 2 Hume Highway Parramatta Road (HW5), Nepean River, Menangle Ashfield Park 4.6m 5 Broadway Harris Street (MR170), Wattle Street (MR594), Westbound travel Broadway Broadway only 4.6m 5 Broadway Wattle Street (MR594), City Road (HW1), Broadway Broadway 4.6m 5 Great Western Church Street (HW5), Western Freeway (M4 Highway Parramatta Western Motorway), Emu Plains 4.6m 5 Great Western Russell Street, Emu Lithgow / Blue Highway Plains Mountains Council Boundary 4.6m 5 Parramatta Road City Road (HW1), Old Canterbury Road Chippendale (MR652), Lewisham 4.6m 5 Parramatta Road George Street, James Ruse Drive Homebush (MR309), Granville 4.6m 5 Parramatta Road James Ruse Drive Marsh Street, Granville No Left Turn (MR309), Granville -

NRMA 2018-19 NSW Budget Submission
2018-19 Budget Submission Prepared for the NSW Government December 2017 2 Table of Contents About the NRMA ..................................................................................................................... 4 Introduction ............................................................................................................................. 5 We were born to keep you moving. ......................................................................................... 7 Addressing cost of living pressures ....................................................................................... 11 2018-19 Budget Recommendations ...................................................................................... 13 2018-19 Budget Priorities ...................................................................................................... 18 Peace of Mind ...................................................................................................................... 18 Road Safety Plan 2021 ...................................................................................................... 18 Road Safety Campaigns .................................................................................................... 18 Connected and Automated Vehicles .................................................................................. 19 Connected and Automated Vehicle Regulations ................................................................ 20 Smart Transport Technology ............................................................................................ -

Closed Areas: Kosciuszko National Park- Part Closure
Closed areas: Kosciuszko National Park - part closure Sections of Kosciuszko National Park have been reopened, but areas which have been burnt or have ongoing fire suppression operations remain closed. Areas Recently Opened: • All areas south of Mt Jagungal and east of the following roads / trails are open to the public: Khancoban – Cabramurra Rd, Alpine Way and Cascade Trail (refer to map). This includes all backcountry areas which have not been impacted by fire. Overnight camping is permitted in these areas. This includes: o Kosciuszko Road - Jindabyne to Charlotte Pass o Guthega Road - Kosciuszko Road to Guthega Village o Charlotte Pass, Perisher Valley, Smiggin Holes, Guthega, Diggers Creek, Wilsons Valley, Sawpit Creek, Waste Point – visitors need to check whether resort facilities and hospitality venues are open for business o Alpine Way between Jindabyne and Thredbo Village o Thredbo Village and Thredbo resort lease area o Ngarigo, Thredbo Diggings and Island Bend campgrounds - for day use and overnight camping o Snowy Mountains Highway – visitors must stay within the road corridor and be aware of hazards such as damaged buildings and burnt trees o Blowering campgrounds - Log Bridge, The Pines, Humes Crossing and Yachting Point o All walks on the Main Range, including to Mt Kosciuszko and surrounding the alpine resort areas o Barry Way – open to the Victorian border. Lower Snowy picnic and campgrounds are open and include; Jacobs River, Halfway Flat, No Name, Pinch River, Jacks Lookout and Running Waters o Schlink Pass trail and associated huts (Horse Camp Hut, White River Hut, Schlink Hut, Valentine Hut) o Mt Jagungal. Access to this area must be from Guthega Power Station or Guthega.