Pollination of the Lady's Slipper Cypripedium Henryi Rolfe
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Pdf of JHOS July 2013
JJoouurrnnaall of the HHAARRDDYY OORRCCHHIIDD SSOOCCIIEETTYY Vol. 10 No. 3 (699) July 2013 JOURNAL of the HARDY ORCHID SOCIETY Vol. 10 No. 3 (69) July 2013 The Hardy Orchid Society Our aim is to promote interest in the study of Native European Orchids and those from similar temperate climates throughout the world. We cover such varied aspects as field study, cultivation and propagation, photography, taxonomy and systematics, and practical conservation. We welcome articles relating to any of these subjects, which will be considered for publication by the editorial committee. Please send your submissions to the Editor, and please structure your text according to the “Advice to Authors” (see website www.hardyorchidsociety.org.uk , January 2004 Journal, Members’ Handbook or contact the Editor). Views expressed in journal arti - cles are those of their author(s) and may not reflect those of HOS. The Hardy Orchid Society Committee President: Prof. Richard Bateman, Jodrell Laboratory, Royal Botanic Gardens Kew, Richmond, Surrey, TW9 3DS Chairman: Celia Wright, The Windmill, Vennington, Westbury, Shrewsbury, Shropshire, SY5 9RG [email protected] Vice-Chairman: vacant Secretary: Richard Robinson, Rhiw, Church Street, Amberley, Sussex, BN18 9NF [email protected] Treasurer: John Wallington, 17, Springbank, Eversley Park Road, London, N21 1JH [email protected] Membership Secretary: Moira Tarrant, Bumbys, Fox Road, Mashbury, Chelmsford, CM1 4TJ [email protected] Plant Show Secretary: David Hughes, Linmoor Cottage, Highwood, -

An Encyclopedia of Shade Perennials This Page Intentionally Left Blank an Encyclopedia of Shade Perennials
An Encyclopedia of Shade Perennials This page intentionally left blank An Encyclopedia of Shade Perennials W. George Schmid Timber Press Portland • Cambridge All photographs are by the author unless otherwise noted. Copyright © 2002 by W. George Schmid. All rights reserved. Published in 2002 by Timber Press, Inc. Timber Press The Haseltine Building 2 Station Road 133 S.W. Second Avenue, Suite 450 Swavesey Portland, Oregon 97204, U.S.A. Cambridge CB4 5QJ, U.K. ISBN 0-88192-549-7 Printed in Hong Kong Library of Congress Cataloging-in-Publication Data Schmid, Wolfram George. An encyclopedia of shade perennials / W. George Schmid. p. cm. ISBN 0-88192-549-7 1. Perennials—Encyclopedias. 2. Shade-tolerant plants—Encyclopedias. I. Title. SB434 .S297 2002 635.9′32′03—dc21 2002020456 I dedicate this book to the greatest treasure in my life, my family: Hildegarde, my wife, friend, and supporter for over half a century, and my children, Michael, Henry, Hildegarde, Wilhelmina, and Siegfried, who with their mates have given us ten grandchildren whose eyes not only see but also appreciate nature’s riches. Their combined love and encouragement made this book possible. This page intentionally left blank Contents Foreword by Allan M. Armitage 9 Acknowledgments 10 Part 1. The Shady Garden 11 1. A Personal Outlook 13 2. Fated Shade 17 3. Practical Thoughts 27 4. Plants Assigned 45 Part 2. Perennials for the Shady Garden A–Z 55 Plant Sources 339 U.S. Department of Agriculture Hardiness Zone Map 342 Index of Plant Names 343 Color photographs follow page 176 7 This page intentionally left blank Foreword As I read George Schmid’s book, I am reminded that all gardeners are kindred in spirit and that— regardless of their roots or knowledge—the gardening they do and the gardens they create are always personal. -

Bee-Mediated Pollen Transfer in Two Populations of Cypripedium Montanum Douglas Ex Lindley
Journal of Pollination Ecology, 13(20), 2014, pp 188-202 BEE-MEDIATED POLLEN TRANSFER IN TWO POPULATIONS OF CYPRIPEDIUM MONTANUM DOUGLAS EX LINDLEY Peter Bernhardt*1, Retha Edens-Meier2, Eric Westhus3, Nan Vance4 1Department of Biology, Saint Louis University, St. Louis, MO 63103, USA 2Department of Educational Studies, Saint Louis University, St. Louis, MO 63103, USA 3Center for Outcomes Research, Saint Louis University, St. Louis, MO 63103, USA 4P.O. Box 282, Kooskia, ID 83539, USA Abstract—The conversion rate of flowers into fruit in C. montanum at two sites over four seasons was 52-85%, unusually high for a food mimic orchid. Comparative measurements of the trap-like labellum of C. montanum showed it was intermediate in size compared to measurements of six other Cypripedium spp. found in North America and China. While visitors to flowers of C. montanum represented three insect orders, at two sites, over four seasons only small- to medium-sized, solitary bees (5-10 mm in length) carried the pollen massulae. Bee-visitation occurred at both sites and began within 24-48 hours following labellum expansion. Female bees in the genus Lasioglossum (Halictidae) were the most common carriers of massulae. However, species of visiting bees differed between sites and years. At both sites the majority of bees entered and escaped from the labellum in less than 180 seconds and there was no significant difference between the times bees spent in the flowers at both sites. At the site on the Eastside Cascades of Central Oregon, there was no correlation between the length and width of a bee and the time it spent escaping from the basal openings. -

Pollination Biology of the Endangered Orchid Cypripedium Japonicum in a Fragmented Forest of Japan
bs_bs_banner Plant Species Biology (2014) 29, 294–299 doi: 10.1111/1442-1984.12016 NOTES AND COMMENTS Pollination biology of the endangered orchid Cypripedium japonicum in a fragmented forest of Japan KENJI SUETSUGU* and SHIGEKI FUKUSHIMA† *Graduate School of Human and Environmental Studies, Kyoto University, Kyoto, and †Chiba Prefectural Agriculture and Forestry Research Center, Sanbu, Japan Abstract Pollination biology studies of the endangered orchid Cypripedium japonicum were conducted in its natural habitat using pollinator observation and hand-pollination experiments. The observed fruit set was as follows: artificial outcross-pollinated, 100%; artificial self-pollinated, 100%; pollinator-excluded, 0%; and emasculated flowers, 0%. These results show that this species, although self-compatible, is neither autogamous nor agamospermous. The fruit set for open-pollinated flowers was 14.9%, which sug- gests that the study population was subject to pollinator limitation. The nectarless flowers of C. japonicum were exclusively visited and pollinated by the queens of two bumblebee species (Bombus ardens and B. diversus diversus). It is probable that the nectarless flowers of C. japonicum attract pollinators through a generalized food decep- tive system. Keywords: Bombus, Cypripedium, deceptive pollination, pollination biology. Received 26 October 2012; revision received 25 February 2013; accepted 1 March 2013 Introduction (Li et al. 2008). As the pollinator passes through the basal orifice, it is forced to pass the stigma and under the The Orchidaceae is one of the most species-rich plant anthers where it picks up some of the pollinium (Li et al. families, and their floral diversity and pollination biology 2008). If the pollinator has already picked up pollen from have long intrigued evolutionary biologists (Cozzolino a previous visit, pollination can also occur under passing & Widmer 2005). -

Universidade Federal De Juiz De Fora Depertamento De Ciências Biológicas Pós-Graduação Em Ciências Biológicas
UNIVERSIDADE FEDERAL DE JUIZ DE FORA DEPERTAMENTO DE CIÊNCIAS BIOLÓGICAS PÓS-GRADUAÇÃO EM CIÊNCIAS BIOLÓGICAS Shaiany Sabrina Lopes Gomes VARIAÇÃO GENÉTICA NO COMPLEXO POLIPLOIDE Zygopetalum maculatum (ORCHIDACEAE) Tese Juiz de Fora, 2017 SHAIANY SABRINA LOPES GOMES VARIAÇÃO GENÉTICA NO COMPLEXO POLIPLOIDE Zygopetalum maculatum (ORCHIDACEAE) Tese de Doutorado do Curso de Pós- Graduação em Ciências Biológicas: Área: Genética e Biotecnologia, para obtenção do Título de Doutora em Ciências Biológicas: Área: Genética e Biotecnologia. Orientador: Lyderson Facio Viccini Coorientadora: Samantha Koehler Juiz de Fora, 2017 Dedicatória Aos meus pais José Pinto Gomes (in memoriam) e Engracia Maria Lopes, exemplos de perseverança, amor infinito, incentivo e dedicação integral. Agradecimentos Minha sincera gratidão, À Coordenação de Aperfeiçoamento Pessoal de Nível Superior (CAPES), pela bolsa de estudo. À Fundação de Amparo à Pesquisa do estado de Minas Gerais (FAPEMIG) e à Fundação de Amparo à Pesquisa do estado de São Paulo (FAPESP) pelo apoio financeiro no projeto e eventos científicos. A Deus, presente em todos os momentos da minha vida, guiando minhas escolhas para o melhor desfecho possível. À Universidade Federal de Juiz de Fora e ao Programa de Pós-graduação em Genética e Biotecnologia, pela oportunidade de realizar o curso para obtenção do Título de doutora em Ciências Biológicas. Aos professores do Programa de Pós-Graduação, pelos ensinamentos. Ao professor doutor Lyderson Facio Viccini, pela orientação neste projeto. Por sua inteira disponibilidade traduzida em ajuda, paciência e grandes ensinamentos. Pela confiança em mim depositada e por ser um exemplo de profissional. À professora doutora Samantha Koehler pela coorientação, além de ceder o material vegetal para o estudo e mostrar-se sempre disposta a colaborações. -

Ecosystem Profile for the Lancang Watershed Submitted by Shanshui
Ecosystem Profile for the Lancang watershed Submitted by Shanshui Conservation Center Drafted by Lei GU, Fangyi YANG, Shan SUN, Ruijuan QI 20 March 2013 1 Content 1. Introduction ............................................................................................................................... 3 2. Biological importance of the Lancang watershed ..................................................................... 4 2.1 Ecology, Climate, Geography, Geology ........................................................................ 5 2.2 Species Diversity ......................................................................................................... 12 2.3 The Protected Area system in the Lancang Watershed................................................ 19 2.4 The Ecosystem Services of the Lancang Watershed ................................................... 24 3. Socioeconomic Context of the Lancang Watershed ................................................................ 27 3.1 Population and Urbanization ....................................................................................... 27 3.2 Society ......................................................................................................................... 29 3.3 Economy ..................................................................................................................... 30 4 An Overview of Current Threats and Their Causes ................................................................ 35 4.1 An Overview of Impacts and Threats ........................................................................ -

Gametophytes and Embryo Ontogeny: Understanding the Reproductive Calendar Of
bioRxiv preprint doi: https://doi.org/10.1101/738799; this version posted August 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Gametophytes and embryo ontogeny: understanding the reproductive calendar of Cypripedium japonicum Thunb. (Cypripedoideae, Orchidaceae) Balkrishna Ghimire, Sung Won Son, Jae Hyun Kim, Mi-Jin Jeong Division of Plant Resources, Korea National Arboretum, Yongmun, 12519, Korea BG: [email protected] (PH: +82-31-5401091) SWS: [email protected] JHK: [email protected] MJJ: [email protected] Date of submission: 17 August, 2019 Noumber of Table: 1 Number of Figures: 8 black and white (color only for online) Word count: 6060 Running title: Gametophytes and embryo ontogeny of Cypripedium japonicum Thunb. Highlight: Manual pollination, reproductive biology and seed development process in Cypripedium japonicum Thunb., a lady’s slipper orchid endemic to East Asia bioRxiv preprint doi: https://doi.org/10.1101/738799; this version posted August 20, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. Abstract Among the flowering plants, the gametophyte development and reproductive biology of orchids is particularly poorly understood. Cypripedium japonicum is a perennial herb, native to East Asia. Due to its limited distribution, the species is included in the Endangered category of the IUCN Red List. Light microscopy and SEM methods were used to study the development of the gametes and embryo. The complete reproductive cycle was developed based on our observations. -

Modelling the Effects of Climate Change on the Distribution of Endangered Cypripedium Japonicum in China
Article Modelling the Effects of Climate Change on the Distribution of Endangered Cypripedium japonicum in China Yadong Xu 1,2, Yi Huang 1,2 , Huiru Zhao 1,2, Meiling Yang 1,2, Yuqi Zhuang 1,2 and Xinping Ye 1,2,* 1 College of Life Sciences, Shaanxi Normal University, Xi’an 710119, China; [email protected] (Y.X.); [email protected] (Y.H.); [email protected] (H.Z.); [email protected] (M.Y.); [email protected] (Y.Z.) 2 Research Center for UAV Remote Sensing, Shaanxi Normal University, Xi’an 710119, China * Correspondence: [email protected]; Tel.: +86-029-8531-0266 Abstract: Cypripedium japonicum is an endangered terrestrial orchid species with high ornamental and medicinal value. As global warming continues to intensify, the survival of C. japonicum will be further challenged. Understanding the impact of climate change on its potential distribution is of great significance to conserve this species. In this study, we established an ensemble species distribution model based on occurrence records of C. japonicum and 13 environmental variables to predict its potential distribution under current and future climatic conditions. The results show that the true skill statistic (TSS), Cohen’s kappa statistic (Kappa), and the area under the receiver operating characteristic curve (AUC) values of the ensemble model were 0.968, 0.906, and 0.995, respectively, providing more robust predictions. The key environmental variables affecting the distribution of C. japonicum were the precipitation in the warmest quarter (Bio18) and the mean temperature in the driest quarter (Bio9). Under future climatic conditions, the total suitable habitat of C. -

Phylogenetics, Genome Size Evolution and Population Ge- Netics of Slipper Orchids in the Subfamily Cypripedioideae (Orchidaceae)
ORBIT - Online Repository of Birkbeck Institutional Theses Enabling Open Access to Birkbecks Research Degree output Phylogenetics, genome size evolution and population ge- netics of slipper orchids in the subfamily cypripedioideae (orchidaceae) http://bbktheses.da.ulcc.ac.uk/88/ Version: Full Version Citation: Chochai, Araya (2014) Phylogenetics, genome size evolution and pop- ulation genetics of slipper orchids in the subfamily cypripedioideae (orchidaceae). PhD thesis, Birkbeck, University of London. c 2014 The Author(s) All material available through ORBIT is protected by intellectual property law, including copyright law. Any use made of the contents should comply with the relevant law. Deposit guide Contact: email Phylogenetics, genome size evolution and population genetics of slipper orchids in the subfamily Cypripedioideae (Orchidaceae) Thesis submitted by Araya Chochai For the degree of Doctor of Philosophy School of Science Birkbeck, University of London and Genetic Section, Jodrell Laboratory Royal Botanic Gardens, Kew November, 2013 Declaration I hereby confirm that this thesis is my own work and the material from other sources used in this work has been appropriately and fully acknowledged. Araya Chochai London, November 2013 2 Abstract Slipper orchids (subfamily Cypripedioideae) comprise five genera; Paphiopedilum, Cypripedium, Phragmipedium, Selenipedium, and Mexipedium. Phylogenetic relationships of the genus Paphiopedilum, were studied using nuclear ribosomal ITS and plastid sequence data. The results confirm that Paphiopedilum is monophyletic and support the division of the genus into three subgenera Parvisepalum, Brachypetalum and Paphiopedilum. Four sections of subgenus Paphiopedilum (Pardalopetalum, Cochlopetalum, Paphiopedilum and Barbata) are recovered with strong support for monophyly, concurring with a recent infrageneric treatment. Section Coryopedilum is also recovered with low bootstrap but high posterior probability values. -
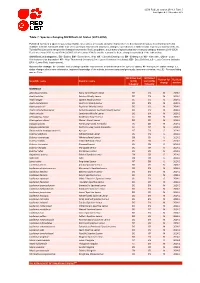
Table 7: Species Changing IUCN Red List Status (2013-2014)
IUCN Red List version 2014.3: Table 7 Last Updated: 13 November 2014 Table 7: Species changing IUCN Red List Status (2013-2014) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2013 (IUCN Red List version 2013.2) and 2014 (IUCN Red List version 2014.3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2013) List (2014) change version Category Category MAMMALS Allocebus trichotis Hairy-eared Dwarf Lemur DD VU N 2014.1 Avahi betsileo Betsileo Woolly Lemur -

CITES and Slipper Orchids
CITES and Slipper Orchids An introduction to slipper orchids covered by the Convention on International Trade in Endangered Species Written by H. Noel McGough, David L. Roberts, Chris Brodie and Jenny Kowalczyk Royal Botanic Gardens, Kew United Kingdom The Board of Trustees, Royal Botanic Gardens, Kew 2006 © The Board of Trustees of the Royal Botanic Gardens, Kew 2006 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form, or by any means, electronic, mechanical, photocopying, recording or otherwise, without written permission of the publisher unless in accordance with the provisions of the Copyright Designs and Patents Act 1988. First published in 2006 by Royal Botanic Gardens, Kew Richmond, Surrey, TW9 3AB, UK www.kew.org ISBN 1-84246-128-1 For information or to purchase Kew titles please visit www.kewbooks.com or email [email protected] Cover image: © Royal Botanic Gardens, Kew CONTENTS Introduction ..................................................................................................... i Acknowledgements ........................................................................................ ii How to Use this Presentation Pack ............................................................... iii References and Resources ........................................................................ iv-ix Slide Index ................................................................................................. x-xi Slides and speaker’s notes ....................................................................... -

3. CYPRIPEDIUM Linnaeus, Sp. Pl. 2: 951. 1753. 杓兰属 Shao Lan Shu Chen Xinqi (陈心启 Chen Sing-Chi); Phillip J
Flora of China 25: 22–33. 2009. 3. CYPRIPEDIUM Linnaeus, Sp. Pl. 2: 951. 1753. 杓兰属 shao lan shu Chen Xinqi (陈心启 Chen Sing-chi); Phillip J. Cribb Arietinum L. C. Beck; Calceolus Miller; Criosanthes Rafinesque; Fissipes Small; Hypodema Reichenbach; Sacodon Rafin- esque. Herbs, with short or long rhizomes and many thickened fibrous roots. Stem erect, elongate or short, clustered or well spaced, often with several sheaths at base. Leaves 1 to several, alternate to opposite, sometimes prostrate on substrate, sheathing and amplexicaul at base; blade adaxially green or sometimes marked with black-purple spots, often elliptic to ovate, rarely cordate or flabellate, with parallel, radiating, or 3–5 prominent veins. Inflorescence terminal, with a solitary flower or rarely many flowers; floral bracts often leaflike, usually smaller than leaves, rarely absent; ovary 1-locular. Flowers usually large and showy. Dorsal sepal erect or hooded over lip; lateral sepals usually united to form a synsepal, but free in Cypripedium plectrochilum. Petals spreading horizontally, at an angle below horizontal, or enfolding sides of lip, sometimes spirally twisted; lip deeply pouched and inflated, subglobose or ellipsoid, with incurved lateral lobes and usually also apical margin, hairy within on bottom. Column short, with 2 lateral fertile stamens, a terminal staminode above, and a stigma below; anthers 2-locular, with very short filaments; pollen powdery or glutinous; staminodes often elliptic to ovate, very rarely ligulate or linear, base stalked or not; stigma ± papillose, inconspicuously 3-lobed. Fruit a capsule. About 50 species: N temperate zone, mainly in temperate Asia and North America, extending south to the Himalayan regions and Central America; 36 species (25 endemic) in China.