Flowering and Fruiting in Cotton
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Polyploidy and the Evolutionary History of Cotton
POLYPLOIDY AND THE EVOLUTIONARY HISTORY OF COTTON Jonathan F. Wendel1 and Richard C. Cronn2 1Department of Botany, Iowa State University, Ames, Iowa 50011, USA 2Pacific Northwest Research Station, USDA Forest Service, 3200 SW Jefferson Way, Corvallis, Oregon 97331, USA I. Introduction II. Taxonomic, Cytogenetic, and Phylogenetic Framework A. Origin and Diversification of the Gossypieae, the Cotton Tribe B. Emergence and Diversification of the Genus Gossypium C. Chromosomal Evolution and the Origin of the Polyploids D. Phylogenetic Relationships and the Temporal Scale of Divergence III. Speciation Mechanisms A. A Fondness for Trans-oceanic Voyages B. A Propensity for Interspecific Gene Exchange IV. Origin of the Allopolyploids A. Time of Formation B. Parentage of the Allopolyploids V. Polyploid Evolution A. Repeated Cycles of Genome Duplication B. Chromosomal Stabilization C. Increased Recombination in Polyploid Gossypium D. A Diverse Array of Genic and Genomic Interactions E. Differential Evolution of Cohabiting Genomes VI. Ecological Consequences of Polyploidization VII. Polyploidy and Fiber VIII. Concluding Remarks References The cotton genus (Gossypium ) includes approximately 50 species distributed in arid to semi-arid regions of the tropic and subtropics. Included are four species that have independently been domesticated for their fiber, two each in Africa–Asia and the Americas. Gossypium species exhibit extraordinary morphological variation, ranging from herbaceous perennials to small trees with a diverse array of reproductive and vegetative -

Genetic Variability Studies in Gossypium Barbadense L
Electronic Journal of Plant Breeding, 1(4): 961-965 (July 2010) Research Article Genetic variability studies in Gossypium barbadense L. genotypes for seed cotton yield and its yield components K. P. M. Dhamayanathi , S. Manickam and K. Rathinavel Abstract A study was carried out during kharif 2006-07 with twenty five Gossypium barbadense L genotypes to obtain information on genetic variability, heritability and genetic advance for seed cotton yield and its yield attributes. Significant differences were observed for characters among genotypes. High genetic differences were recorded for nodes/plant, sympodia, bolls as well as fruiting points per plant, seed cotton yield, lint index indicating ample scope for genetic improvement of these characters through selection. Results also revealed high heritability coupled with high genetic advance for yield and most of the yield components as well as fibre quality traits. Sympodia/plant, fruiting point /plant, number of nodes/plant, number of bolls per plant, and lint index were positively correlated with seed cotton yield per plant and appeared to be interrelated with each other. It is suggested that these characters could be considered as selection criteria in improving the seed cotton yield of G. barbadense , L genotypes. Key words : Gossypium barbadense , genetic variability, heritability, genetic advance, lint index, selection criteria Introduction Seed cotton yield is a complex trait governed by Cotton is the most widely used vegetable fibre and several yield contributing characters such as plant also the most important raw material for the textile height, number of monopodia, number of industry, grown in tropical and subtropical regions sympodia, number of bolls, number of fruiting in more than 80 countries all over the world. -

The Argo Navis Constellation
THE ARGO NAVIS CONSTELLATION At the last meeting we talked about the constellation around the South Pole, and how in the olden days there used to be a large ship there that has since been subdivided into the current constellations. I could not then recall the names of the constellations, but remembered that we talked about this subject at one of the early meetings, and now found it in September 2011. In line with my often stated definition of Astronomy, and how it seems to include virtually all the other Philosophy subjects: History, Science, Physics, Biology, Language, Cosmology and Mythology, lets go to mythology and re- tell the story behind the Argo Constellation. Argo Navis (or simply Argo) used to be a very large constellation in the southern sky. It represented the ship The Argo Navis ship with the Argonauts on board used by the Argonauts in Greek mythology who, in the years before the Trojan War, accompanied Jason to Colchis (modern day Georgia) in his quest to find the Golden Fleece. The ship was named after its builder, Argus. Argo is the only one of the 48 constellations listed by the 2nd century astronomer Ptolemy that is no longer officially recognised as a constellation. In 1752, the French astronomer Nicolas Louis de Lacaille subdivided it into Carina (the keel, or the hull, of the ship), Puppis (the poop deck), and Vela (the sails). The constellation Pyxis (the mariner's compass) occupies an area which in antiquity was considered part of Argo's mast (called Malus). The story goes that, when Jason was 20 years old, an oracle ordered him to head to the Iolcan court (modern city of Volos) where king Pelias was presiding over a sacrifice to Poseidon with several neighbouring kings in attendance. -

Assessment of Gene Flow Between Gossypium Hirsutum and G
G3: Genes|Genomes|Genetics Early Online, published on May 25, 2017 as doi:10.1534/g3.117.041509 Assessment of gene flow between Gossypium hirsutum and G. herbaceum: evidence of unreduced gametes in the diploid progenitor. Montes E *1, Coriton O †, Eber F †, Huteau V †, Lacape JM ‡, Reinhardt C §2, Marais D §, Hofs JL **, Chèvre AM † 2, Pannetier C * ‡ 2 *: Institut Jean-Pierre Bourgin, INRA, AgroParisTech, CNRS, Université Paris-Saclay, RD10, F-78026 Versailles Cedex, France †: Institut de Génétique, Environnement et Protection des Plantes, INRA, Agrocampus Ouest, Université de Rennes I., BP35327, 35653 Le Rheu, France ‡: CIRAD, UMR AGAP, Amélioration Génétique et Adaptation des Plantes méditerranéennes et tropicales, 34398 Montpellier, France. §: Department of Plant and Soil Sciences, University of Pretoria, Pretoria 0001, South Africa ** : CIRAD, UR AIDA, Agro-écologie et Intensification Durable des cultures Annuelles, 34398 Montpellier, France. 1 present address: UMR 1349, IGEPP, INRA BP35327, 35653 Le Rheu, France 2 corresponding authors Running title Unreduced gametes in diploid cotton 1 © The Author(s) 2013. Published by the Genetics Society of America. Key words Gene flow, natural hybridization, unreduced gamete, Gossypium hirsutum, Gossypium herbaceum Corresponding authors: UMR 1349, Institut de Génétique, Environnement et Protection des Plantes, Institut National de la Recherche Agronomique (INRA), BP35327, F-35653 Le Rheu, France. Email [email protected] and UMR1318, Institut Jean-Pierre Bourgin, INRA F-78026 Versailles, France. Email [email protected]. 2 Abstract In the framework of a gene flow assessment, we investigated the natural hybridization rate between Gossypium hirsutum (AADD genome) and G. herbaceum (AA genome). The latter species, a diploid progenitor of G. -

A Global Assembly of Cotton Ests
Downloaded from genome.cshlp.org on October 3, 2021 - Published by Cold Spring Harbor Laboratory Press Resource A global assembly of cotton ESTs Joshua A. Udall,1 Jordan M. Swanson,1 Karl Haller,2 Ryan A. Rapp,1 Michael E. Sparks,1 Jamie Hatfield,2 Yeisoo Yu,3 Yingru Wu,4 Caitriona Dowd,4 Aladdin B. Arpat,5 Brad A. Sickler,5 Thea A. Wilkins,5 Jin Ying Guo,6 Xiao Ya Chen,6 Jodi Scheffler,7 Earl Taliercio,7 Ricky Turley,7 Helen McFadden,4 Paxton Payton,8 Natalya Klueva,9 Randell Allen,9 Deshui Zhang,10 Candace Haigler,10 Curtis Wilkerson,11 Jinfeng Suo,12 Stefan R. Schulze,13 Margaret L. Pierce,14 Margaret Essenberg,14 HyeRan Kim,3 Danny J. Llewellyn,4 Elizabeth S. Dennis,4 David Kudrna,3 Rod Wing,3 Andrew H. Paterson,13 Cari Soderlund,2 and Jonathan F. Wendel1,15 1Department of Ecology, Evolution, and Organismal Biology, Iowa State University, Ames, Iowa 50011, USA; 2Arizona Genomics Computational Laboratory, BIO5 Institute, 3Arizona Genomics Institute, Department of Plant Sciences, University of Arizona, Tucson, Arizona 85721, USA; 4CSIRO Plant Industry, Canberra City ACT 2601, Australia; 5Department of Plant Sciences, University of California–Davis, Davis, California 95616, USA; 6Institute of Plant Physiology and Ecology, Shanghai Institutes for Biological Sciences, Shanghai, 200032, China; 7United States Department of Agriculture–Agricultural Research Service, Stoneville, Mississippi 38776, USA; 8United States Department of Agriculture–Agricultural Research Service, Lubbock, Texas 79415, USA; 9Department of Biology, Texas Tech University, -

Malvales Nymphaeales Austrobaileyales
Amborellales Malvales Nymphaeales Austrobaileyales Acorales G Eenzaadlobbigen G Alismatales Petrosaviales Huerteales Pandanales Een recente ontwikkeling is het Dioscoreales Dipentodontaceae in een nieuw Liliales Asparagales hout- en anatomische kenmerke 2 geslachten en 5 soorten van b Arecales en samengestelde bladeren, die G Commeliniden G Dasypogonales Poales werden geplaatst. De Dipentod Commelinales sinicus, een boom uit China en Zingiberales die vroeger in de Violales werd Ceratophyllales Malvales Chloranthales De Malvales zijn voor het meren Canellales warme streken. Ze hebben vers Piperales G Magnoliiden G De bast is nogal eens vezelig, st Magnoliales veel voor. De kroonbladen ligge Laurales Ze hebben meestal een lange st Ranunculales De zaden en de binnenkant van Sabiales bezet. Deze orde omvatte al de Proteales Trochodendrales Dipterocarpaceae, Bixaceae, Ne Buxales Sphaerosepalaceae. De Lindefam Gunnerales Bombacaceae zijn nu opgenom Berberidopsidales (Malvaceae). De Muntingiaceae Dilleniales afgesplitst. Nieuwkomers in de Caryophyllales Santalales (Cistaceae), uit de Violales, en d Saxifragales (Thymelaeaceae) uit de Euphorb Cytinaceae (vroeger Rafflesiales G Geavanceerde tweezaadlobbigen G Vitales Crossosomatales ook in deze orde thuis. Geraniales Myrtales Sapindales Zygophyllales De meeste soorten in deze orde Celastrales houtige gewassen, vaak met sam Malpighiales G Fabiden G Oxalidales Fabales Rosales Bixaceae G Rosiden G Cucurbitales Malvaceae Fagales Muntingiaceae Cistaceae Huerteales Dipterocarpaceae G G Malviden Brassicales -

Larly in Gossypium
CHROMOSOMES IN GOSSYPIUM AND RELATED GENERA^ By A. E. LoNGLEy2 Associate Botanist, Division of Genetics and Biophysics, Bureau of Plant Industry, United States Department of Agriculture INTRODUCTION An investigation of the chromosomes in the Malvaceae, particu- larly in Gossypium, was undertaken because of the repeated failures of cotton breeders in their attempts to hybridize such cottons as Garo Hill {Gossypium cernuum Tod.) with any of the varieties of upland cotton (G. hirsutum L.). In 1923 a study was made of the chromosomes in a few repre- sentative species of Gossypium growing in the greenhouses at Wash- ington, D. C. Among these were found some species with 13 and some with 26 as their haploid chromosome number. Neither number corresponded with that found earlier by Cannon (4) ^ or Balls (1). Simultaneously with two other investigators, Denham (5, 6), of England, and Nikoljeva as reported by Zaitzev (15, 16), of Russia, the writer found that cultivated varieties of Gossypium fall into two classes, those with 13 chromosomes and those with 26 chromosomes. This classification corresponds with the taxonomic separation of Gos- sypium species into the Old World, or Asiatic, and the New World, or American, groups. MATERIAL AND METHODS The present investigation has been confined to pollen mother cells during the reduction phases. Young buds were selected and the staminal column removed and put at once into Bouin^s, Carnoy's, or chromo-acetic (1:1:100) killing solution, embedded, sectioned, and stained. It was soon found that very satisfactory preparations of pollen mother cells in which the chromosomes were dividing could be made from fresh material stained in aceto-carmine fluid. -

Cotton: the Fabric of Our Lives
Cotton: The Fabric of Our Lives By Angela Box Oils, balls, swabs, bandages, tissue, paper, napkins, diapers, socks, underwear, shirts, shorts, sweaters, pants, coats, towels, linen, cushions, drapery, upholstery, rugs, carpet, comforters, mattresses, insulation, filtration, and many other things that are used daily by everyone are composed of, or inspired by cotton. Cotton is a soft, fluffy, naturally occurring fiber plant that can be processed into an array of materials and goods. Many, many things that we wear, sleep on, sleep under, walk on, or utilize in wound-care, etc., contain some percentage of cotton. It is a fiber that is used everyday, by everyone, in one way or another. It has qualities that have made it a choice crop for centuries around the world. Today though, cotton is being largely displaced by synthetic fibers that have qualities that exceed the natural crop plant. These fibers can also be mass-produced and sold at relatively lower costs. Still, cotton stands alone as the most utilized fiber crop plant used around the world. Also known as "King Cotton," in the United States, it was the major force behind the institution of the American age of slavery, and cotton prevailed as the economic source for the southern states of the United States and its antebellum prosperity before the civil war. It holds an important place in America's past, present, and future. Cotton is truly the "Fabric of Our Lives". Characteristics Cotton is an annual, biennial or perennial plant, but in cultivation it is generally treated as an annual; herbaceous to short shrub or small tree - two to six feet tall. -
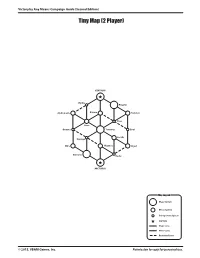
Tiny Map (2 Player)
Victory by Any Means Campaign Guide (Second Edition) Tiny Map (2 Player) CENTAURI Hydra Regulus Andromeda Baham Rotanev Naos Siren Gemma Terminus Errai Lesath Kapteyn Mira Phaeton Algol Canopus Hadar ARCTURUS Map Legend Major System Minor System Unimportant System CAPITAL Major Lane Minor Lane Restricted Lane © 2015, VBAM Games, Inc. Permission to copy for personal use. Victory by Any Means Campaign Guide (Second Edition) Small Map (3 Player) CENTAURI Scorpius Rigel Zaurak Canopus Regulus Sualocin Cassiopeia Perseus Errai Malus Menkar Algol Dorado Chara Bessel Sadatoni Ankaa Pegasus Terminus Cayrel Celaeno Tania Theemin Sabik Aries Vega Sheliak Aquila DRACONIS ORION Phoenix Tigris Herschel Sirius Ksora Aldebaran Map Legend Major System Minor System Unimportant System CAPITAL Major Lane Minor Lane Restricted Lane © 2015, VBAM Games, Inc. Permission to copy for personal use. Victory by Any Means Campaign Guide (Second Edition) Medium Map (4 Player) Canopus Cerberus Betria Vulpecula Pegasus Luyten ORION CENTAURI Sualocin Kapteyn Mintaka Mizar Phaeton Rana Altair Lilium Leo Rangifer Ruchba Thuban Mira Asterion Sabik Ksora Nihal Spica Sulafat Alshat Pavonis Sagittarius Lyra Terminus Capella Sarin Gemma Heka Hercules Hadar Taygeta Bootes Rotanev Geidi Noctua Algol Phoenix Errai Regulus Fomalhaut Tigris Alrischa Aquila Dorado Ankaa ARCTURUS ANTARES Eridanus Lesath Perseus Celaeno Zaurak Map Legend Sirius Major System Minor System Unimportant System CAPITAL Major Lane Minor Lane Restricted Lane © 2015, VBAM Games, Inc. Permission to copy for personal -

U.S. EPA, Pesticides, Label, PENNCOZEB 4FL FLOWABLE
UNITED'STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, D.C. 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION Mr. Ross Gilbert JUL 1 2. a110 . Pyxis Regulatory Consulting, Inc. For United Phosphorus, Inc. th 4110 13 . St. NW Gig Harbor, W A 98332 SUBJECT: Application for Pesticide Notification (PRN 98-10) Request Warranty Statement EPA Reg. No. 70506-194 Application Dated June 9, 2010 Dear Registrant: The Agency is in receipt of your Application for Pesticide Notification under Pesticide Registration Notice (PRN) 98-10 dated 06/09/10 for the above product. The Registration Division (RD) has conducted a review of this request for its applicability under PRN 98-10 and finds that· the action(s) requested fall within the scope ofPRN 98-10. The label submitted with the application has been stamped ''Notification'' and will be placed in our records. If you have any questions, please call me directly at 703-305-5335 or Owen F. Beeder of my staff at 703-308-8899. Sincerely, 1 " 12 W,c>U (I.!ji{tfYN/~ Paul 1. Matrradone, Ph.D., Acting ~ Notifications & Minor Formulations 'Nam Leader Registration Division (7505P) Office of Pesticide Programs PfU$e re.d i".tnH:tiD~n fflvene befDffI comp/etjntl fDrm. Form ADDroved •. OMB No. 2070.oo8ll __XDir .. 2·28-95 opp Identifier Number United States WRegistration &EPA Environmental Prolection Agency Amendment Washington, DC 20460 : .; Other ; Application for Pesticide - Section I 1. Company/Product Number 2. EPA Product Manager 3. Proposed Classification 70506-184 M. Waller o Nonlt D Restricted 4. Company/Product (Name) PM' United Phosphorus, Inc. -

Checklist of the Washington Baltimore Area
Annotated Checklist of the Vascular Plants of the Washington - Baltimore Area Part I Ferns, Fern Allies, Gymnosperms, and Dicotyledons by Stanwyn G. Shetler and Sylvia Stone Orli Department of Botany National Museum of Natural History 2000 Department of Botany, National Museum of Natural History Smithsonian Institution, Washington, DC 20560-0166 ii iii PREFACE The better part of a century has elapsed since A. S. Hitchcock and Paul C. Standley published their succinct manual in 1919 for the identification of the vascular flora in the Washington, DC, area. A comparable new manual has long been needed. As with their work, such a manual should be produced through a collaborative effort of the region’s botanists and other experts. The Annotated Checklist is offered as a first step, in the hope that it will spark and facilitate that effort. In preparing this checklist, Shetler has been responsible for the taxonomy and nomenclature and Orli for the database. We have chosen to distribute the first part in preliminary form, so that it can be used, criticized, and revised while it is current and the second part (Monocotyledons) is still in progress. Additions, corrections, and comments are welcome. We hope that our checklist will stimulate a new wave of fieldwork to check on the current status of the local flora relative to what is reported here. When Part II is finished, the two parts will be combined into a single publication. We also maintain a Web site for the Flora of the Washington-Baltimore Area, and the database can be searched there (http://www.nmnh.si.edu/botany/projects/dcflora). -

Frequencylistsforvocab
FREQUENCY LISTS FOR VOCABULARY DRILL There are three ofthese lists: (1) Words found 12—23 times in the first six books of the Aeneid (pp. 97—100); (2) Words found 6—11 times in the first six books (pp. 10 1—106); (3) General Word List: Words found twenty-four times or more in the first six books (pp. 108—111 and pull-out, inside of back cover). WORDS FOUND 12-23 TIMES IN AENEID 1-6 abeo go away, depart aureus of gold, gold(en) acer sharp, piercing auris EAR addo give to, ADD auster south (wind) aequö (make) level, EQUALIZE autem moreover, however aether upper air, ETHER auxilium support, assistance affor speak to, address Averto turn from, AVERT agnosco RECOGNIZE, understand Ala wing bis twice aliquis some (one), any (one) alter other (of two), second cadö fall, die amicus friend(ly), AMICABLE caecus blind, unseeing amittö lose, send away canö sing (of), CHANT amö love, cherish carina keel, ship an whether cärus dear annus year causa CAUSE, reason antrum cave, cavern cavus hollow aperio uncover, open cêdö yield, CEDE arbor tree celer swift arduus steep, ARDUOUS celsus high, lofty ars ART, skill centum one hundred artus (noun) joint, limb certamen contest asper rough, harsh certus settled, CERTAIN aspicio look (at), behold cingö encircle, gird asto stand (by, near) cinis ashes, CINDERS astrum star, constellation clams CLEAR, famous attollö lift (up), arouse colO CULTIVATE, worship audeo be eager, dare coma hair, foliage (of a tree) 97 98 WORD LISTS WORD LISTS 99 condo put together, found fortis brave, strong litus broad, wide opus work;