Possible Relationship Between Vocal Communication System and Fat Reserve in Wintering Birds: a Test of the Optimal Body Mass Theory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Complete Species Table in Species Number Order
Page 1 of 19 Complete Species Table in Species Number order Go to species 100 .0, 200 .0, 300 .0, 400 .0, 500 .0, 600 .0, 700 .0, 800 .0, 900 .0 SPECIES COMMON NAME ALPHA CODE BAND SIZE 001 .0 Western Grebe WEGR 7A 7B 001 .1 Clark's Grebe CLGR 7A 7B 002 .0 Red-necked Grebe RNGR 7A 003 .0 Horned Grebe HOGR 6 5 004 .0 Eared Grebe EAGR 5 005 .0 Least Grebe LEGR 4 006 .0 Pied-billed Grebe PBGR 5 6 007 .0 Common Loon COLO 8 008 .0 Yellow-billed Loon YBLO 9 009 .0 Arctic Loon ARLO 7B 010 .0 Pacific Loon PALO 7B 011 .0 Red-throated Loon RTLO 7B 012 .0 Tufted Puffin TUPU 6 5 013 .0 Atlantic Puffin ATPU 5 014 .0 Horned Puffin HOPU 5 015 .0 Rhinoceros Auklet RHAU 5 6 016 .0 Cassin's Auklet CAAU 3B-3A 017 .0 Parakeet Auklet PAAU 4 018 .0 Crested Auklet CRAU 4 019 .0 Whiskered Auklet WHAU 3 020 .0 Least Auklet LEAU 2 3 021 .0 Ancient Murrelet ANMU 3B 3 023 .0 Marbled Murrelet MAMU 3B 3 023 .1 Long-billed Murrelet LBMU 3B 3 024 .0 Kittlitz's Murrelet KIMU 3B 025 .0 Xantus's Murrelet XAMU 2 026 .0 Craveri's Murrelet CRMU 2 027 .0 Black Guillemot BLGU 4 029 .0 Pigeon Guillemot PIGU 4A 030 .0 Common Murre COMU 6M 031 .0 Thick-billed Murre TBMU 6M 5R 032 .0 Razorbill RAZO 5R 034 .0 Dovekie DOVE 3 035 .0 Great. -

Checklist of Maine Birds
Black-throated Blue Warbler Snow Bunting Yellow-rumped Warbler CARDINALS and ALLIES (CARDINALIDAE) Black-throated Green Warbler Northern Cardinal Blackburnian Warbler Rose-breasted Grosbeak Pine Warbler Blue Grosbeak Prairie Warbler Indigo Bunting Palm Warbler Dickcissel Bay-breasted Warbler BLACKBIRDS (ICTERIDAE) Field Checklist of Maine Birds Blackpoll Warbler Bobolink Black-and-white Warbler Red-winged Blackbird American Redstart Eastern Meadowlark Date & Location Birders Ovenbird Yellow-headed Blackbird ___________________________ Northern Waterthrush Rusty Blackbird Louisiana Waterthrush Common Grackle ___________________________ Mourning Warbler Brown-headed Cowbird ___________________________ Common Yellowthroat Orchard Oriole Wilson’s Warbler Baltimore Oriole ___________________________ Canada Warbler Pine Grosbeak Yellow-breasted Chat Purple Finch TANAGERS (THRAUPIDAE) House Finch GEESE and DUCKS (TINAMIDAE) Ruffed Grouse Summer Tanager Red Crossbill Snow Goose Spruce Grouse Scarlet Tanager White-winged Crossbill Canada Goose Wild Turkey NEW WORLD SPARROWS (EMBERIZIDAE) Common Redpoll Brant LOONS (GAVIIDAE) Eastern Towhee Pine Siskin Tundra Swan Red-throated Loon American Tree Sparrow American Goldfinch Wood Duck Pacific Loon Chipping Sparrow Evening Grosbeak Gadwall Common Loon Clay-colored Sparrow OLD WORLD SPARROWS (PASSERIDAE) American Wigeon GREBES (PODICIPEDIDAE) Field Sparrow House Sparrow American Black Duck Pied-billed Grebe Lark Sparrow ADDITIONAL SPECIES Mallard Horned Grebe Vesper Sparrow Blue-winged Teal Red-necked -

L O U I S I a N A
L O U I S I A N A SPARROWS L O U I S I A N A SPARROWS Written by Bill Fontenot and Richard DeMay Photography by Greg Lavaty and Richard DeMay Designed and Illustrated by Diane K. Baker What is a Sparrow? Generally, sparrows are characterized as New World sparrows belong to the bird small, gray or brown-streaked, conical-billed family Emberizidae. Here in North America, birds that live on or near the ground. The sparrows are divided into 13 genera, which also cryptic blend of gray, white, black, and brown includes the towhees (genus Pipilo), longspurs hues which comprise a typical sparrow’s color (genus Calcarius), juncos (genus Junco), and pattern is the result of tens of thousands of Lark Bunting (genus Calamospiza) – all of sparrow generations living in grassland and which are technically sparrows. Emberizidae is brushland habitats. The triangular or cone- a large family, containing well over 300 species shaped bills inherent to most all sparrow species are perfectly adapted for a life of granivory – of crushing and husking seeds. “Of Louisiana’s 33 recorded sparrows, Sparrows possess well-developed claws on their toes, the evolutionary result of so much time spent on the ground, scratching for seeds only seven species breed here...” through leaf litter and other duff. Additionally, worldwide, 50 of which occur in the United most species incorporate a substantial amount States on a regular basis, and 33 of which have of insect, spider, snail, and other invertebrate been recorded for Louisiana. food items into their diets, especially during Of Louisiana’s 33 recorded sparrows, Opposite page: Bachman Sparrow the spring and summer months. -

Winter Bird Highlights 2015, Is Brought to You by U.S
Winter Bird Highlights FROM PROJECT FEEDERWATCH 2014–15 FOCUS ON CITIZEN SCIENCE • VOLUME 11 Focus on Citizen Science is a publication highlight- FeederWatch welcomes new ing the contributions of citizen scientists. This is- sue, Winter Bird Highlights 2015, is brought to you by U.S. project assistant Project FeederWatch, a research and education proj- ect of the Cornell Lab of Ornithology and Bird Studies Canada. Project FeederWatch is made possible by the e are pleased to have a new efforts and support of thousands of citizen scientists. Wteam member on board! Meet Chelsea Benson, a new as- Project FeederWatch Staff sistant for Project FeederWatch. Chelsea will also be assisting with Cornell Lab of Ornithology NestWatch, another Cornell Lab Janis Dickinson citizen-science project. She will Director of Citizen Science be responding to your emails and Emma Greig phone calls and helping to keep Project Leader and Editor the website and social media pages Anne Marie Johnson Project Assistant up-to-date. Chelsea comes to us with a back- Chelsea Benson Project Assistant ground in environmental educa- Wesley Hochachka tion and conservation. She has worked with schools, community Senior Research Associate organizations, and local governments in her previous positions. Diane Tessaglia-Hymes She incorporated citizen science into her programming and into Design Director regional events like Day in the Life of the Hudson River. Chelsea holds a dual B.A. in psychology and English from Bird Studies Canada Allegheny College and an M.A. in Social Science, Environment Kerrie Wilcox and Community, from Humboldt State University. Project Leader We are excited that Chelsea has brought her energy and en- Rosie Kirton thusiasm to the Cornell Lab, where she will no doubt mobilize Project Support even more people to monitor bird feeders (and bird nests) for Kristine Dobney Project Assistant science. -

Federal Register/Vol. 85, No. 74/Thursday, April 16, 2020/Rules
21282 Federal Register / Vol. 85, No. 74 / Thursday, April 16, 2020 / Rules and Regulations DEPARTMENT OF THE INTERIOR United States and the Government of United States or U.S. territories as a Canada Amending the 1916 Convention result of recent taxonomic changes; Fish and Wildlife Service between the United Kingdom and the (8) Change the common (English) United States of America for the names of 43 species to conform to 50 CFR Part 10 Protection of Migratory Birds, Sen. accepted use; and (9) Change the scientific names of 135 [Docket No. FWS–HQ–MB–2018–0047; Treaty Doc. 104–28 (December 14, FXMB 12320900000//201//FF09M29000] 1995); species to conform to accepted use. (2) Mexico: Convention between the The List of Migratory Birds (50 CFR RIN 1018–BC67 United States and Mexico for the 10.13) was last revised on November 1, Protection of Migratory Birds and Game 2013 (78 FR 65844). The amendments in General Provisions; Revised List of this rule were necessitated by nine Migratory Birds Mammals, February 7, 1936, 50 Stat. 1311 (T.S. No. 912), as amended by published supplements to the 7th (1998) AGENCY: Fish and Wildlife Service, Protocol with Mexico amending edition of the American Ornithologists’ Interior. Convention for Protection of Migratory Union (AOU, now recognized as the American Ornithological Society (AOS)) ACTION: Final rule. Birds and Game Mammals, Sen. Treaty Doc. 105–26 (May 5, 1997); Check-list of North American Birds (AOU 2011, AOU 2012, AOU 2013, SUMMARY: We, the U.S. Fish and (3) Japan: Convention between the AOU 2014, AOU 2015, AOU 2016, AOS Wildlife Service (Service), revise the Government of the United States of 2017, AOS 2018, and AOS 2019) and List of Migratory Birds protected by the America and the Government of Japan the 2017 publication of the Clements Migratory Bird Treaty Act (MBTA) by for the Protection of Migratory Birds and Checklist of Birds of the World both adding and removing species. -

Illinois Birds: Volume 4 – Sparrows, Weaver Finches and Longspurs © 2013, Edges, Fence Rows, Thickets and Grain Fields
ILLINOIS BIRDS : Volume 4 SPARROWS, WEAVER FINCHES and LONGSPURS male Photo © Rob Curtis, The Early Birder female Photo © John Cassady Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Mary Kay Rubey Photo © Rob Curtis, The Early Birder American tree sparrow chipping sparrow field sparrow vesper sparrow eastern towhee Pipilo erythrophthalmus Spizella arborea Spizella passerina Spizella pusilla Pooecetes gramineus Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder lark sparrow savannah sparrow grasshopper sparrow Henslow’s sparrow fox sparrow song sparrow Chondestes grammacus Passerculus sandwichensis Ammodramus savannarum Ammodramus henslowii Passerella iliaca Melospiza melodia Photo © Brian Tang Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Photo © Rob Curtis, The Early Birder Lincoln’s sparrow swamp sparrow white-throated sparrow white-crowned sparrow dark-eyed junco Le Conte’s sparrow Melospiza lincolnii Melospiza georgiana Zonotrichia albicollis Zonotrichia leucophrys Junco hyemalis Ammodramus leconteii Photo © Brian Tang winter Photo © Rob Curtis, The Early Birder summer Photo © Rob Curtis, The Early Birder Photo © Mark Bowman winter Photo © Rob Curtis, The Early Birder summer Photo © Rob Curtis, The Early Birder Nelson’s sparrow -

BLESS Bird Guide to Lois Hole Centennial Provincial Park
Bird Guide to Lois Hole Centennial Provincial Park, Alberta Big Lake Environment Support Society Credits Technical information Most of the information on bird species was reprinted with permission from the Cornell Lab of Ornithology’s website AllAboutBirds.org, some text came from Wikipedia, and some was modified for the Big Lake region of Alberta. Photographs Local photographers were approached for good quality images, and where good photographs were not available then freely available images from Wikipedia were used (see page 166 for individual photo credits). Funding City of St. Albert, Environmental Initiatives Grant Administration and Review Miles Constable Big Lake Environment Support Society Produced by Big Lake Environment Support Society P.O. Box 65053 St. Albert, Ab T8N 5Y3 www.bless.ab.ca For information contact [email protected] 2 Bird Guide to Lois Hole Centennial Provincial Park, Alberta 2016 3 Location of Lois Hole Centennial Provincial Park, Alberta Map courtesy of Google, Inc. There are a great many birds to be seen in Lois Hole Centennial Provincial Park as Big Lake has been designated an Important Bird Area. This Guide features the most commonly seen birds; however, it is not a complete guide to all birds that could be seen at Big Lake. If you are, or become, passionate about birds, we recommend a comprehensive guide to the birds of North America as there are many species that migrate through Big Lake, or that are expanding their range into this area for a variety of reasons. There are also simply those individuals that wander off course and end up in our area; those wonderful lost individuals that keep birders on their toes. -

Dear Eastbay Volunteer
Area Search Species Codes Species Codes Please use the species codes on the list below. The full list is online at: http://www.pwrc.usgs.gov/bbl/manual/bandsize.htm Species Numbers, Common Names, Alpha Codes SPECIES COMMON NAME ALPHA CODE 001.0 Western Grebe WEGR 001.1 Clark's Grebe CLGR 002.0 Red-necked Grebe RNGR 003.0 Horned Grebe HOGR 004.0 Eared Grebe EAGR 005.0 Least Grebe LEGR 006.0 Pied-billed Grebe PBGR 007.0 Common Loon COLO 008.0 Yellow-billed Loon YBLO 009.0 Arctic Loon ARLO 010.0 Pacific Loon PALO 011.0 Red-throated Loon RTLO 012.0 Tufted Puffin TUPU 013.0 Atlantic Puffin ATPU 014.0 Horned Puffin HOPU 015.0 Rhinoceros Auklet RHAU 016.0 Cassin's Auklet CAAU 017.0 Parakeet Auklet PAAU 018.0 Crested Auklet CRAU 019.0 Whiskered Auklet WHAU 020.0 Least Auklet LEAU 021.0 Ancient Murrelet ANMU 023.0 Marbled Murrelet MAMU 023.1 Long-billed Murrelet LBMU 024.0 Kittlitz's Murrelet KIMU 025.0 Xantus's Murrelet XAMU 026.0 Craveri's Murrelet CRMU 027.0 Black Guillemot BLGU 029.0 Pigeon Guillemot PIGU 030.0 Common Murre COMU 031.0 Thick-billed Murre TBMU 032.0 Razorbill RAZO 034.0 Dovekie DOVE 035.0 Great Skua GRSK 035.2 South Polar Skua SPSK 036.0 Pomarine Jaeger POJA 037.0 Parasitic Jaeger PAJA 038.0 Long-tailed Jaeger LTJA 039.0 Ivory Gull IVGU 039.1 Magellan Gull MAGU 040.0 Black-legged Kittiwake BLKI 041.0 Red-legged Kittiwake RLKI 042.0 Glaucous Gull GLGU 043.0 Iceland Gull ICGU 043.1 Thayer's Gull THGU 044.0 Glaucous-winged Gull GWGU 044.6 Hybrid Gull HYGU 047.0 Great Black-backed Gull GBBG 048.0 Slaty-backed Gull SBGU 049.0 Western -

Fernald Preserve Bird List
Bird List Order: ANSERIFORMES Order: PODICIPEDIFORMES Order: GRUIFORMES Order: COLUMBIFORMES Family: Anatidae Family: Podicipedidae Family: Rallidae Family: Columbidae Black-bellied Whistling Duck Pied-billed Grebe Virginia Rail Rock Pigeon Greater White-fronted Goose Horned Grebe Sora Mourning Dove Snow Goose Red-necked Grebe Common Gallinule Order: CUCULIFORMES Ross’s Goose American Coot Order: SULIFORMES Family: Cuculidae Cackling Goose Family: Gruidae Family: Phalacrocoracidae Yellow-billed Cuckoo Canada Goose Sandhill Crane Mute Swan Double-crested Cormorant Black-billed Cuckoo Trumpeter Swan Order: CHARADRIIFORMES Order: PELECANIFORMES Order: STRIGIFORMES Tundra Swan Family: Recurvirostridae Family: Ardeidae Family: Tytonidae Wood Duck Black-necked Stilt American White Pelican Barn Owl Gadwall Family: Charadriidae American Bittern Family: Strigidae Eurasian Wigeon Black-bellied Plover Least Bittern Eastern Screech-Owl American Wigeon American Golden Plover Great Blue Heron Great Horned Owl American Black Duck Semipalmated Plover Great Egret Barred Owl Mallard Killdeer Snowy Egret Long-eared Owl Blue-winged Teal Family: Scolopacidae Little Blue Heron Short-eared Owl Norther Shoveler Spotted Sandpiper Cattle Egret Northern Saw-whet Owl Northern Pintail Solitary Sandpiper Green Heron Garganey Greater Yellowlegs Order: CAPRIMULGIFORMES Black-crowned Night-Heron Green-winged Teal Willet Family: Caprimulgidae Canvasback Family: Threskiornithidae Lesser Yellowlegs Common Nighthawk Glossy Ibis Redhead Upland Sandpiper Ring-necked Duck White-faced -

Winter Bird Highlights
Winter Bird Highlights FROM PROJECT FEEDERWATCH 2018–19 FOCUS ON CITIZEN SCIENCE • VOLUME 15 Focus on Citizen Science is a publication highlight- A FeederWatcher photo on the ing the contributions of citizen scientists. This is- sue, Winter Bird Highlights 2019, is brought to you by cover of a new book! Project FeederWatch, a research and education pro- ject of the Cornell Lab of Ornithology and Bird Studies Canada. Project FeederWatch is made possible by the n upcoming book, Wildlife Disease Ecology, efforts and support of thousands of citizen scientists. features a chapter Project FeederWatch Staff by Cornell Lab or- WILDLIFE DISEASE ECOLOGY A Linking Theory to Data and Application Cornell Lab of Ornithology nithologists and House Finch Edited by Kenneth Wilson, Andy Fenton and Dan Tompkins Emma Greig eye disease researchers André Project Leader and Editor Anne Marie Johnson Dhondt and Wes Hochachka. Project Assistant According to the publisher, Holly Faulkner Project Assistant Cambridge University Press, David Bonter “Each chapter in the book in- Director of Citizen Science Wesley Hochachka troduces a host and disease Senior Research Associate and explains how that system has aided our general Bird Studies Canada understanding of the evolution and spread of wildlife Kerrie Wilcox diseases, through the development and testing of im- Project Leader Rosie Kirton portant epidemiological and evolutionary theories.” Project Support The book’s cover (pictured above) has a photo of a Kristine Dobney Project Assistant House Finch with eye disease taken by FeederWatcher Jody Allair Director of Citizen Science Gary Mueller. The book is scheduled for publication in Denis Lepage November. -

Iowa Species of Greatest Conservation
Iowa Breeding Bird Species of Greatest Conservation Need Species highlighted in bold are documented to nest in Iowa urban areas Status Habitat Preference Common Name Scientific Name Cygnus 1 Trumpeter Swan Large prairie marshes w/emergent vegetation buccinator 2 American Wigeon Anas americana Prairie marshes w/upland vegetation; wetlands 3 Blue -winged Teal Anas discors Marshes, wetlands and shallow ponds 4 Northern Pintail Anas acuta Prairie marshes w/upland vegetation 5 Canvasback Aythya valisineria Bulrush & cattail prairie marsh 6 Redhead Aythya americana Cattail & bulrush hemi-marshes Cattail & bulrush hemi-marshes, small lakes 7 Ring -necked Duck Aythya collaris and ponds 8 Lesser Scaup Aythya affinis Cattail & bulrush hemi-marshes Colinus 9 Northern Bobwhite Prefers medium ht. grasslands w/shrubs & forbs virginianus Prefers large forests or woodlands containing 10 Ruffed Grouse Bonasa umbellus areas of high stem density Tympanuchus 11 ** Sharp-tailed Grouse Open prairie w/patches of shrubs or trees phasianellus Tympanuchus 12 Greater Prairie-Chicken Prefers large grassland tracts +2,000 acres cupido Podiceps Cattail & bulrush hemi-marshes, vegetated 13 Red -necked Grebe grisegena shallow lakes Podiceps Cattail & bulrush hemi-marshes, vegetated 14 Eared Grebe nigricollis shallow lakes Pelecanus Reservoirs, impoundments, rivers Riparian 15 American White Pelican erythrorhynchos areas w/islands-for nesting Botaurus 16 American Bittern Large prairie marshes w/upland grassland lentiginosus Black-crowned Night- Nycticorax Prairie marshes -
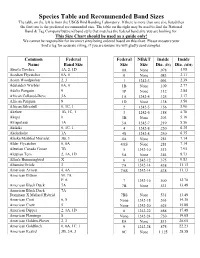
Species Table and Recommended Band Sizes the Table on the Left Is from the USGS Bird Banding Laboratory
Species Table and Recommended Band Sizes The table on the left is from the USGS Bird Banding Laboratory. If there is more than one size listed then the first one is the preferred recommended size. The table on the right may be used to find the National Band & Tag Company butt-end band style that matches the federal band size you are looking for. This Size Chart should be used as a guide only! We cannot be responsible for incorrect sizes being ordered based on this chart. Please measure your bird’s leg for accurate sizing, if you are unsure we will gladly send samples. Common Federal Federal NB&T Inside Inside Name Band Size Size Size Dia. (IN) Dia. (MM) Abert's Towhee 1A, 2, 1D 0A None .078 1.98 Acadian Flycatcher 0A, 0 0 None .083 2.11 Acorn Woodpecker 2, 3 1 1242-3 .094 2.39 Adelaide's Warbler 0A, 0 1B None .109 2.77 Adelie Penguin 9 1P None .112 2.84 African Collared-Dove 3A 1A 1242-4 .125 3.17 African Penguin 9 1D None .138 3.50 African Silverbill 0, 1C, 1 2 1242-5 .156 3.96 Akekee 1B, 1C, 1 3 1242-6 .188 4.78 Akepa 0 3B None .203 5.16 Akiapolaau 1A 3A 1242-7 .219 5.56 Akikiki 0, 1C, 1 4 1242-8 .250 6.35 Akohekohe 1A 4S 1242-8 .250 6.35 Alaska Marbled Murrelet 3B, 3 4A None .281 7.14 Alder Flycatcher 0, 0A 4AS None .281 7.14 Aleutian Canada Goose 7B 5 1242-10 .313 7.95 Aleutian Tern 2, 1A, 1D 5A None .344 8.73 Allen's Hummingbird X 6 1242-12 .375 9.53 Altamira Oriole 3 7A 1242-14 .438 11.13 American Avocet 4, 4A 7AS 1242-14 .438 11.13 American Bittern M: 7A F: 6 7 1242-16 .500 12.70 American Black Duck 7A 7B None .531 13.49 American