An Elevated Igg4 Response in Chronic Infectious Aortitis Is Associated with Aortic Atherosclerosis Zakir Siddiquee1, R Neal Smith1 and James R Stone1,2
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Cogan's Syndrome
IMAGING IN MEDICINE COLODETTI R ET AL. Cogan’s syndrome – A rare aortitis, difficult to diagnose but with therapeutic potential RAIZA COLODETTI1, GUILHERME SPINA2, TATIANA LEAL3, MUCIO OLIVEIRA JR4, ALEXANDRE SOEIRO3* 1MD Cardiologist, Instituto do Coração (InCor), Hospital das Clínicas, Faculdade de Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil 2Assistant Physician at the Valvular Heart Disease Outpatient Clinic, InCor, HC-FMUSP, São Paulo, SP, Brazil 3Assistant Physician at the Clinical Emergency Service, InCor, HC-FMUSP, São Paulo, SP, Brazil 4Director of the Clinical Emergency Service, InCor, HC-FMUSP, São Paulo, SP, Brazil SUMMARY Study conducted at Unidade Clínica de Emergência, Instituto do Coração (InCor), The inflammation of aortic wall, named aortitis, is a rare condition that can Hospital das Clínicas, Faculdade de be caused by a number of pathologies, mainly inflammatory or infectious in Medicina da Universidade de São Paulo (HC-FMUSP), São Paulo, SP, Brazil nature. In this context, the occurrence of combined audiovestibular and/or ocular manifestations eventually led to the diagnosis of Cogan’s syndrome, Article received: 8/18/2017 Accepted for publication: 9/9/2017 making it the rare case, but susceptible to adequate immunosuppressive treatment and satisfactory disease control. *Correspondence: Address: Av. Dr. Enéas de Carvalho Aguiar, 44 Keywords: chest pain, aortitis, Cogan’s syndrome. São Paulo, SP – Brazil Postal code: 05403-900 [email protected] http://dx.doi.org/10.1590/1806-9282.63.12.1028 INTRODUCTION four years ago the episodes began to intensify. He was Inflammation of the aortic wall, called aortitis, is an in- admitted to another service a week before for the same frequent clinical condition that manifests itself with sys- reason, where he underwent coronary angiography, show- temic symptoms and may cause precordial pain.1-4 One ing no coronary obstruction, and an echocardiogram, of the rheumatologic causes of aortitis is a rare disease which revealed a slight dilatation of the ascending aorta. -

Noninfectious Ascending Aortitis: Staying Ahead of the Curve
Editorial Noninfectious Ascending Aortitis: Staying Ahead of the Curve Aortitis is the general name for a spectrum of disorders aortic aneurysms is common with aortitis, the vast majority involving inflammation of the aorta1. The causes of aortitis of aortic aneurysms, both thoracic and abdominal, are due are most easily sorted into either infectious or noninfectious to noninflammatory disease including cystic medial degen- disease. Infections associated with aortitis include syphilis, eration, atherosclerosis, inherited connective tissue dis- Salmonella, Staphylococcus, other bacteria, and mycobacte- eases, or a combination of these processes8. ria, especially M. tuberculosis. Noninfectious causes of aor- The most common serious clinical sequelae of aortitis titis include several forms of primary and secondary are thoracic aortic aneurysms, especially of the ascending large-vessel vasculitis. Giant cell arteritis (GCA) and aorta. The rate of aortic aneurysms among patients with Takayasu’s arteritis are the most common causes of nonin- aortitis is markedly elevated compared to the general popu- fectious aortitis. At least 20% of patients with GCA and lation, especially for ascending thoracic lesions. The reason 50%–70% of patients with Takayasu’s arteritis will develop for this differential tropism for the proximal versus distal changes consistent with aortitis, many of whom develop aorta in aortitis, which is the opposite of what is seen in aortic aneurysms2-7. Aortic involvement is also seen in noninflammatory aortic aneurysms, is not clear but may be relapsing polychondritis, Cogan’s syndrome, and Behçet’s related to differences in the tissue structure, presence of disease, and has been reported as a rare association with vasa vasorum, blood flow dynamics, and other factors9. -

Survival After Aortic Dissection in Giant Cell Arteritis
332 Letters to the editor pressure or hypotensive episodes were technique and rating scale for abnormalities of the thorax, without intravenous contrast seen in scleroderma and related disorders. recorded during the trial. Among the side Arthritis Rheum 1981; 24: 1159-65. medium (because of asthma), showed a effects, mild flushing (35%) and headache 9 Ferri C, La Civita L, Zignego A et al. Plasma normal mediastinum but a small rim of L, Ann Rheum Dis: first published as 10.1136/ard.55.5.332 on 1 May 1996. Downloaded from (24%) were the most frequent in isradipine levels of endothelin-I in mixed cryoglobulin- pleural fluid on the left. aemia patients. Br J Rheumatol 1994; 33: recipients. 689-90. At follow up, the patient complained of This study has demonstrated the favour- 10 Yanagisawa M, Kurihara H, Kimura S, et al. A right thigh claudication. Radiofemoral delay able effects of isradipine on patients with novel potent vasoconstrictor peptide pro- was noted. CT of the abdomen showed Raynaud's phenomenon; moreover, it is the duced by vascular endothelial cells. Nature internal displacement ofcalcified atheroma in 1988; 332: 411-5. first to demonstrate a significant reduction 11 Levin E R. Endothelins [review]. N Engl J Med the aorta, suggesting aortic dissection. Mag- in plasma concentrations of ET-1 during 1995; 333: 356-63. netic resonance imaging (MRI) confirmed calcium antagonist treatment. The most this as distal to the origin of the left sub- usual manifestations of Raynaud's phenom- clavian artery, extending to the aortic bifur- enon are pain and numbness in the fingers, cation. -

Statistical Analysis Plan
Cover Page for Statistical Analysis Plan Sponsor name: Novo Nordisk A/S NCT number NCT03061214 Sponsor trial ID: NN9535-4114 Official title of study: SUSTAINTM CHINA - Efficacy and safety of semaglutide once-weekly versus sitagliptin once-daily as add-on to metformin in subjects with type 2 diabetes Document date: 22 August 2019 Semaglutide s.c (Ozempic®) Date: 22 August 2019 Novo Nordisk Trial ID: NN9535-4114 Version: 1.0 CONFIDENTIAL Clinical Trial Report Status: Final Appendix 16.1.9 16.1.9 Documentation of statistical methods List of contents Statistical analysis plan...................................................................................................................... /LQN Statistical documentation................................................................................................................... /LQN Redacted VWDWLVWLFDODQDO\VLVSODQ Includes redaction of personal identifiable information only. Statistical Analysis Plan Date: 28 May 2019 Novo Nordisk Trial ID: NN9535-4114 Version: 1.0 CONFIDENTIAL UTN:U1111-1149-0432 Status: Final EudraCT No.:NA Page: 1 of 30 Statistical Analysis Plan Trial ID: NN9535-4114 Efficacy and safety of semaglutide once-weekly versus sitagliptin once-daily as add-on to metformin in subjects with type 2 diabetes Author Biostatistics Semaglutide s.c. This confidential document is the property of Novo Nordisk. No unpublished information contained herein may be disclosed without prior written approval from Novo Nordisk. Access to this document must be restricted to relevant parties.This -

Peripheral Vascular Disease Imaging Guidelines
Cigna Medical Coverage Policies – Radiology Peripheral Vascular Disease (PVD) Imaging Guidelines Effective October 1, 2021 ____________________________________________________________________________________ Instructions for use The following coverage policy applies to health benefit plans administered by Cigna. Coverage policies are intended to provide guidance in interpreting certain standard Cigna benefit plans and are used by medical directors and other health care professionals in making medical necessity and other coverage determinations. Please note the terms of a customer’s particular benefit plan document may differ significantly from the standard benefit plans upon which these coverage policies are based. For example, a customer’s benefit plan document may contain a specific exclusion related to a topic addressed in a coverage policy. In the event of a conflict, a customer’s benefit plan document always supersedes the information in the coverage policy. In the absence of federal or state coverage mandates, benefits are ultimately determined by the terms of the applicable benefit plan document. Coverage determinations in each specific instance require consideration of: 1. The terms of the applicable benefit plan document in effect on the date of service 2. Any applicable laws and regulations 3. Any relevant collateral source materials including coverage policies 4. The specific facts of the particular situation Coverage policies relate exclusively to the administration of health benefit plans. Coverage policies are not recommendations for treatment and should never be used as treatment guidelines. This evidence-based medical coverage policy has been developed by eviCore, Inc. Some information in this coverage policyy m nota ap ply to all benefit plans administered by Cigna. These guidelines include procedures eviCore does not review for Cigna. -

NIH Public Access Author Manuscript Circulation
NIH Public Access Author Manuscript Circulation. Author manuscript; available in PMC 2009 October 9. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: Circulation. 2008 June 10; 117(23): 3039±3051. doi:10.1161/CIRCULATIONAHA.107.760686. Aortitis Heather L. Gornik, M.D., M.H.S. and Assistant Professor of Medicine, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University Medical Director, Non-Invasive Vascular Laboratory Department of Cardiovascular Medicine The Cleveland Clinic Foundation 9500 Euclid Avenue/Desk S60 Cleveland, Ohio 44120 (216) 445-3689 [email protected] Mark A. Creager, M.D.* Professor of Medicine, Harvard Medical School Simon C. Fireman Scholar in Cardiovascular Medicine Director, Vascular Center Brigham and Women's Hospital 75 Francis Street Boston, Massachusetts 02115 (617) 732-5267 [email protected] Keywords Aortitis; vasculitis; giant cell arteritis; Takayasu arteritis; aortic aneurysm Aortitis is the all-encompassing term ascribed to inflammation of the aorta. The most common causes of aortitis are the large vessel vasculitides, giant cell arteritis (GCA) and Takayasu arteritis, although it is also associated with several other rheumatologic diseases. Infectious aortitis is a rare but potentially life-threatening disorder. In some cases, aortitis is an incidental finding at the time of histopathologic examination following surgery for aortic aneurysm. As the clinical presentation of aortitis is highly variable, the cardiovascular clinician must have a high index of suspicion to establish an accurate diagnosis of this disorder in a timely fashion. In this manuscript, we review the pathophysiology, epidemiology, diagnostic approach, and management of aortitis. -

Retroperitoneal Fibrosis and Aortitis As the Initial Findings of Systemic Lupus Erythematosus
Turk J Rheumatol 2011;26(3):262-264 doi: 10.5152/tjr.2011.042 Case Report Retroperitoneal Fibrosis and Aortitis as the Initial Findings of Systemic Lupus Erythematosus Sistemik Lupus Eritematozusun Başlangıç Bulguları Olarak Retroperitoneal Fibrozis ve Aortitis Aynur SOYUÖZ,1 Metin IŞIK,2 İsmail DOĞAN,2 Levent KILIÇ,2 Sedat KİRAZ2 1Department of Internal Medicine, Medical Faculty of Hacettepe University, Ankara, Turkey; 2Department of Internal Medicine, Division of Rheumatology, Medical Faculty of Hacettepe University, Ankara, Turkey Retroperitoneal fibrosis (RPF) is characterized by Retroperitoneal fibrozis (RPF) progressif bağ doku progressive connective tissue proliferation which in proliferasyonu ile karakterize olup nadiren periaortik kitle rare cases forms a periaortic mass. Aortitis is clinically oluşturur. Aortitis sistemik lupus eritematozusta (SLE) uncommon in systemic lupus erythematosus (SLE), and klinik olarak nadirdir ve literatürde daha önce yaşayan only one previous case with aortitis has been reported in lupuslu yetişkin hastalarda yalnızca bir aortitis olgusu the literature in living adult patients with lupus. Herein, we bildirilmiştir. Burada, başlangıç SLE bulguları RPF ve report a case with RPF and aortitis as the initial findings aortitis olan bir SLE olgusu sunuldu. Hasta kliniğimize of SLE. The patient was admitted to our clinic with severe her iki avuç içinde ağrılı ve makülopapüler lezyonlarla abdominal pain, nausea, and photosensitivity along with birlikte şiddetli karın ağrısı, bulantı ve fotosensitivite ile -

Sjögren's Syndrome with Polyserositis, Gastrointestinal Findings And
: Ope gy n A lo c o c i e g s n s A Heper et al., Angiol 2017, 5:2 Angiology: Open Access DOI: 10.4172/2329-9495.1000193 ISSN: 2329-9495 Case Report Open Access Sjögren’s Syndrome with Polyserositis, Gastrointestinal Findings and Ascending Aortic Aneurysm Gulumser Heper, MD1*, Suha Cetin, MD1, Kemal Unal, MD2, Salih Salihi, MD3, Basak Bostancı, MD4, Murat Korkmaz, MD5 1Department of Cardiology, Okan University Medical Faculty, Istanbul, Turkey 2Department of Nuclear Medicine, Okan University Medical Faculty, Istanbul, Turkey 3Department of Cardiovascular Surgery, Okan University Medical Faculty, Istanbul, Turkey 4Department of Ophthalmology, Okan University Medical Faculty, Istanbul, Turkey 5Department of Gastroenterology, Okan University Medical Faculty, Istanbul, Turkey *Corresponding author: Gulumser Heper, Department of Cardiology, Okan University, Aydınlı Cad No:2 34947 İçmeler, Tuzla, Istanbul, Turkey, Tel: 00905325252999; E-mail: [email protected] Received date: 27 April, 2017; Accepted date: 05 May, 2017; Published date: 09 May, 2017 Copyright: © 2017 Heper G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. Abstract Sjögren’s syndrome (SS) is an autoimmune disease with glandular and extraglandular manifestations. Pleural and pericardial effusions in association with SS are rare. Similarly, ascites is rare and it can occur in SS when combined with primary biliary cirrhosis (PBC). Inflammatory Abdominal Aortic Aneurysm together with SS has been described only in one case. We report herein the case of a 70-year-old man with SS presenting with polyserositis (pleural and pericardial effusion and ascites) and gastrointestinal manifestations (atrophic gastritis and candida esophagitis) and ascending aorta aneurysm. -

A Rare but Potentially Lethal Case of Tuberculous Aortic Aneurysm Presenting with Repeated Attacks of Abdominal Pain
□ CASE REPORT □ A Rare but Potentially Lethal Case of Tuberculous Aortic Aneurysm Presenting with Repeated Attacks of Abdominal Pain Yao-Min Hung 1-3, Yun-Te Chang 1-3, Jyh-Seng Wang 2,4, Paul Yung-Pou Wang 5 and Shue-Ren Wann 1-3 Abstract Tuberculous aortic aneurysm is an extremely rare disease with a high mortality rate. The clinical features of this condition are highly variable, ranging from asymptomatic with or without constitutional symptoms, abdominal pain to frank rupture, bleeding and shock. We herein report the case of a 56-year-old man with a large tuberculous mycotic aneurysm in the abdominal aorta with an initial presentation of repeated attacks of abdominal pain lasting for several months. Due to the vague nature of the initial symptoms, tuberculous aor- tic aneurysms may take several months to diagnose. This case highlights the importance of having a high in- dex of suspicion and providing timely surgery for this rare but potentially lethal disease. Key words: abdominal aortic aneurysm, abdominal pain, aneurysm, aorta, mycotic aneurysm, tuberculosis (Intern Med 54: 1145-1148, 2015) (DOI: 10.2169/internalmedicine.54.3620) a 56-year-old man who presented to the ED with repeated Introduction attacks of abdominal pain. Following a preoperative evalu- ation, including clinical and radiological assessments, he un- Tuberculosis (TB) remains a major global health problem. derwent successful repair of a mycotic aneurysm in the ab- This disease causes ill-health in millions of people each year dominal aorta with excision and replacement of the diseased and ranks as the second leading cause of death from infec- aorta. -
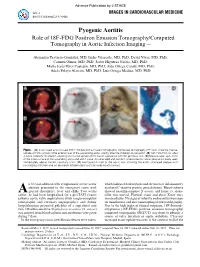
Pyogenic Aortitis ― Role of 18F-FDG Positron Emission Tomography/Computed Tomography in Aortic Infection Imaging ―
Advance Publication by-J-STAGE Circ J IMAGES IN CARDIOVASCULAR MEDICINE doi: 10.1253/circj.CJ-17-0455 Pyogenic Aortitis ― Role of 18F-FDG Positron Emission Tomography/Computed Tomography in Aortic Infection Imaging ― Alejandro Travieso-González, MD; Isidre Vilacosta, MD, PhD; David Vivas, MD, PhD; Carmen Olmos, MD, PhD; Javier Higueras Nafría, MD, PhD; María Jesús Pérez Castejón, MD, PhD; Aída Ortega Candil, MD, PhD; Adela Pelayo Alarcón, MD, PhD; Luis Ortega Medina, MD, PhD Figure. (A) Axial fused and unfused 18F-FDG positron emission tomography computed tomography (PET/CT) showing intense uptake of FDG (arrow) in the anterior wall of the ascending aorta, shortly after the initiation of cloxacillin. (B) 18F-FDG PET-CT after 4-week antibiotic treatment, showing increased uptake of the tracer compared with the previous one. (C) Macroscopic specimen of the inner surface of the ascending aorta and aortic valve. An ulcerated and necrotic atherosclerotic aortic plaque is clearly seen immediately above the left coronary sinus. (D) Hematoxylin stain of the aortic wall showing the aortic ulcerated plaque with necrotizing infection and an abundant inflammatory reaction underneath (arrow). n 85-year-old man with symptomatic severe aortic which indicated leukocytosis and elevation of inflammatory stenosis presented to the emergency room with markers (C-reactive protein, procalcitonin). Blood cultures A general discomfort, fever and chills. Two weeks showed oxacillin-sensitive S. aureus, and hence i.v. cloxa- earlier, he had been hospitalized for a pre-TAVI (trans- cillin was started. Physical exam and chest X-ray were catheter aortic valve implatation) study (angio-computed unremarkable. No signs of infective endocarditis were seen tomography and coronary angiography), and during on transthoracic and also transesophageal echocardiography. -
Consensus Statement on Surgical Pathology of the Aorta, Part I
Cardiovascular Pathology 24 (2015) 267–278 Contents lists available at ScienceDirect Cardiovascular Pathology Review Article Consensus statement on surgical pathology of the aorta from the Society for Cardiovascular Pathology and the Association for European Cardio- vascular Pathology: I. Inflammatory diseases James R. Stone a,⁎,PatrickBrunevalb,⁎⁎, Annalisa Angelini c, Giovanni Bartoloni d, Cristina Basso c, Lubov Batoroeva e,L.MaximilianBujaf, Jagdish Butany g, Giulia d'Amati h, John T. Fallon i, Adriana C. Gittenberger-de Groot j, Rosa H. Gouveia k, Marc K. Halushka l, Karen L. Kelly m, Ivana Kholova n, Ornella Leone o, Silvio H. Litovsky p, Joseph J. Maleszewski q, Dylan V. Miller r, Richard N. Mitchell s, Stephen D. Preston t,AngelaPucciu, Stanley J. Radio v,E.ReneRodriguezw, Mary N. Sheppard x, S. Kim Suvarna y, Carmela D. Tan w, Gaetano Thiene c, Allard C. van der Wal z, John P. Veinot aa a Massachusetts General Hospital, Boston, MA, USA b University Paris-Descartes, France c University of Padua Medical School, Padua, Italy d University of Catania, Catania, Italy e Russian Academy of Medical Sciences, Irkutsk, Russia f University of Texas Health Science Center at Houston, Houston, TX, USA g Toronto General Hospital, Toronto, Canada h Sapienza, University of Rome, Rome, Italy i New York Medical College, Valhalla, NY, USA j Leiden University, Leiden, the Netherlands k Hospital Santa Cruz, Carnaxide, Portugal l Johns Hopkins University, Baltimore, MD, USA m East Carolina University, Greenville, NC, USA n Fimlab Laboratories, Tampere, -

Insights Into Imaging of Aortitis
Insights into Imaging of Aortitis The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Litmanovich, Diana, Afra Yıldırım, and Alexander Bankier. 2012. Insights into imaging of aortitis. Insights into Imaging 3(6): 545-560. Published Version doi:10.1007/s13244-012-0192-x Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:10611838 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Other Posted Material, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#LAA Insights Imaging (2012) 3:545–560 DOI 10.1007/s13244-012-0192-x REVIEW Insights into imaging of aortitis Diana E. Litmanovich & Afra Yıldırım & Alexander A. Bankier Received: 20 May 2012 /Revised: 8 August 2012 /Accepted: 9 August 2012 /Published online: 20 September 2012 # The Author(s) 2012. This article is published with open access at Springerlink.com Abstract both the initial diagnosis and follow-up. Conclusion: This Background Aortitis is a subtype of the more general term review focusses on the most common manifestations of “vasculitis”, an inflammatory condition of infectious or aortitis with which radiologists should be familiar. noninfectious origin involving the vessel wall. The term Teaching Points “vasculitis” refers to a broad spectrum of diseases with • Aortitis is an inflammatory condition of infectious/nonin- different aetiologies, pathophysiologies, clinical presenta- fectious origin involving the vessel wall. tions and prognoses. The clinical manifestations are nonspe- • Imaging findings can help in the assessment of aortitis and cific, as are the laboratory findings such as pain, fever, are often crucial for the final diagnosis.