COVID-19 Vaccine Astrazeneca Analysis Print
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Taste Alteration in COVID-19: a Rapid Review with Data Synthesis Reveals Significant Geographical Differences
medRxiv preprint doi: https://doi.org/10.1101/2020.09.11.20192831; this version posted September 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license . Taste alteration in COVID-19: a rapid review with data synthesis reveals significant geographical differences By Nicola Cirillo Melbourne Dental School, The University of Melbourne, 3053 Carlton, Victoria, Australia *Address for Correspondence: Prof. Dr. Nicola Cirillo Melbourne Dental School The University of Melbourne 720 Swanston Street, Carlton 3053 Victoria, AUS Tel/fax. +61 03 9341 1473 e-mail: [email protected] NOTE: This preprint reports new research that has not been certified by peer review and should not be used to guide clinical practice. medRxiv preprint doi: https://doi.org/10.1101/2020.09.11.20192831; this version posted September 13, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted medRxiv a license to display the preprint in perpetuity. It is made available under a CC-BY 4.0 International license . Abstract To facilitate a timely understanding of the differences in the prevalence of gustatory disturbances (GD) in individuals infected with SARS-CoV-2, we undertook a rapid systematic review of articles published in the repository of the National Library of Medicine (MEDLINE/PubMed) and medRxiv from their inception until September 3, 2020. The minimum requirements for completing a restricted systematic review were met. -

How to Manage 'Breast' Pain
How to Manage ‘Breast’ Pain. Breast pain is the commonest presenting breast complaint to GPs and the commonest reason for referral to the Breast Unit. Nearly 70% of women develop breast pain at some point in their lives but in only 1% of patients with true breast pain is it related to breast cancer. It is however a major source of worry and anxiety for patients, with many convinced they have breast cancer. The anxiety caused can perpetuate the symptoms and for some lead to psychological morbidity such as loss of self‐esteem and depression. Breast pain can be divided into cyclical and non‐cyclical breast pain with non‐cyclical being divided into true breast pain and referred pain. Cyclical breast pain Younger women often present because of an increase or change in the pain they normally experience before or during a period. Seventy‐five per cent of women with breast pain have cyclical breast pain worse around the time of menstruation. This is linked to changes in hormone levels and mainly affects premenopausal women. It may be associated with heaviness, tenderness, pricking or stabbing pains and can affect one or both breasts or the axillae. This type of pain is common and often self‐limiting. It usually stops after the menopause unless HRT is taken. Non‐cyclical breast pain Non‐cyclical breast pain is continuous pain not related to the menstrual cycle. It is either true breast pain or extra mammary pain that feels as if it is coming from the breast. The majority of non‐cyclical pain seen in clinic is non‐breast and originates from the chest wall e.g. -
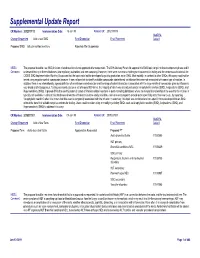
Detail Report
Supplemental Update Report CR Number: 2012319113 Implementation Date: 16-Jan-19 Related CR: 2012319113 MedDRA Change Requested Add a new SMQ Final Disposition Final Placement Code # Proposed SMQ Infusion related reactions Rejected After Suspension MSSO The proposal to add a new SMQ Infusion related reactions is not approved after suspension. The ICH Advisory Panel did approve this SMQ topic to go into the development phase and it Comment: underwent testing in three databases (two regulatory authorities and one company). However, there were numerous challenges encountered in testing and the consensus decision of the CIOMS SMQ Implementation Working Group was that the topic could not be developed to go into production as an SMQ. Most notably, in contrast to other SMQs, this query could not be tested using negative control compounds because it was not possible to identify suitable compounds administered via infusion that were not associated with some type of reaction. In addition, there is no internationally agreed definition of an infusion related reaction and the range of potential reactions associated with the large variety of compounds given by infusion is very broad and heterogenous. Testing was conducted on a set of around 500 terms, the majority of which was already included in Anaphylactic reaction (SMQ), Angioedema (SMQ), and Hypersensitivity (SMQ). It proved difficult to identify potential cases of infusion related reactions in post-marketing databases where the temporal relationship of the event to the infusion is typically not available. In clinical trial databases where this information is more easily available, users are encouraged to provide more specificity about the event, e.g., by reporting “Anaphylactic reaction” when it is known that this event is temporally associated with the infusion. -

Vasospasm of the Nipple
Vasospasm of the Nipple A spasm of blood vessels (vasospasm) in the nipple can result in nipple and/or breast pain, particularly within 30 minutes after a breastfeeding or a pumping session. It usually happens after nipple trauma and/or an infection. Vasospasms can cause repeated disruption of blood flow to the nipple. Within seconds or minutes after milk removal, the nipple may turn white, red, or purple, and a burning or Community stabbing pain is felt. Occasionally women feel a tingling sensation or itching. As the Breastfeeding nipple returns to its normal color, a throbbing pain may result. Color change is not Center always visible. 5930 S. 58th Street If there is a reason for nipple damage (poor latch or a yeast overgrowth), the cause (in the Trade Center) Lincoln, NE 68516 needs to be addressed. This can be enough to stop the pain. Sometimes the (402) 423-6402 vasospasm continues in a “vicious” cycle, as depicted below. While the blood 10818 Elm Street vessels are constricted, the nipple tissue does not receive enough oxygen. This Rockbrook Village causes more tissue damage, which can lead to recurrent vasospasm, even if the Omaha, NE 68144 (402) 502-0617 original cause of damage is “fixed.” For additional information: (Poor Latch or Inflammation) www ↓ Tissue Damage ↙ ↖ Spasm of blood vessels → Lack of oxygen to tissues To promote improved blood flow and healing of the nipple tissue: • See a lactation consultant (IBCLC) or a breastfeeding medicine specialist for help with latch and/or pumping to reduce future nipple damage. • When your baby comes off your nipple, or you finish a pumping session, immediately cover your nipple with a breast pad or a towel to keep it warm and dry. -

SPRAVATO® Esketamine Hydrochloride DATA SHEET
SPRAVATO® esketamine hydrochloride DATA SHEET 1. PRODUCT NAME SPRAVATO® esketamine hydrochloride 32.3mg (equivalent to 28mg esketamine) nasal spray 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each single use nasal spray device delivers two sprays, one spray into each nostril. Total volume of drug product per device to be delivered is 0.2 mL containing a total of 32.3 mg of esketamine hydrochloride (equivalent to 28 mg of esketamine). For the full list of excipients, see Section 6.1 List of Excipients. 3. PHARMACEUTICAL FORM Nasal spray, solution. Clear, colourless, aqueous solution. 4. CLINICAL PARTICULARS 4.1 Therapeutic Indications SPRAVATO in conjunction with an oral antidepressant, is indicated for: - treatment resistant depression (Major Depressive Disorder in adults who have not responded adequately to at least two different antidepressants of adequate dose and duration to treat the current depressive episode). - rapid reduction of depressive symptoms in patients with Major Depressive Disorder who have acute suicidal ideation or behaviour. SPRAVATO is not indicated to prevent suicide or in reducing suicidal ideation or behaviour (see section 4.4. Special Warnings And Precautions For Use) 4.2 Dose and method of administration In patients with TRD, SPRAVATO should be administered in conjunction with a newly initiated oral antidepressant (AD) therapy. In patients with MDSI, SPRAVATO should be administered in conjunction with standard of care therapy. During the Phase III MDSI clinical program, standard of care oral AD treatment (either AD monotherapy or AD plus augmentation therapy) was initiated or optimised. Important Considerations Prior to Initiating and During Therapy SPRAVATO must be administered under the direct supervision of a healthcare provider. -

Second Edition
COVID-19 Evidence Update COVID-19 Update from SAHMRI, Health Translation SA and the Commission on Excellence and Innovation in Health Updated 4 May 2020 – 2nd Edition “What is the prevalence, positive predictive value, negative predictive value, sensitivity and specificity of anosmia in the diagnosis of COVID-19?” Executive Summary There is widespread reporting of a potential link between anosmia (loss of smell) and ageusia (loss of taste) and SARS-COV-2 infection, as an early sign and with sudden onset predominantly without nasal obstruction. There are calls for anosmia and ageusia to be recognised as symptoms for COVID-19. Since the 1st edition of this briefing (25 March 2020), there has been a significant expansion of literature on this topic, including 3 systematic reviews. Predictive value: The reported prevalence of anosmia/hyposmia and ageusia/hypogeusia in SARS-COV-2 positive patients are in the order of 36-68% and 33-71% respectively. There are reports of anosmia as the first symptom in some patients. Estimates from one study for hyposmia and hypogeusia: • Positive likelihood ratios: 4.5 and 5.8 • Sensitivity: 46% and 62% • Specificity: 90% and 89% The US Centres for Disease Control and Prevention (CDC) has added new loss or taste or smell to its list of recognised symptoms for SARS-COV-2 infection. To date, the World Health Organization has not. Conclusion: There is sufficient evidence to warrant adding loss of taste and smell to the list of symptoms for COVID-19 and promoting this information to the public. Context • Early detection of COVID-19 is key to the ongoing management of the pandemic. -

Unifying the Motor & Non-Motor Features of Parkinson's Disease
Unifying the Motor & Non-motor Features of Parkinson’s Disease Ali Shalash Professor of Neurology Chair of Ain Shams Movement Disorders Group Department of Neurology, Ain Shams Univeristy Cairo, Egypt AGENDA 1. Motor and non-motor Symptoms 2. Pathophysiology of Motor & NMS 3. Relation To Disease Course 4. NMS in motor subtypes 5. Motor & NMS fluctuation 6. Treatment of Motor & NMS: links Parkinson’s Disease • PD prevalence 1 % of populations older than 65 years (Abbas et al., 2018) • From 1990 to 2015, the number of with PD patients doubled to over 6 million, and double again to over 12 million by 2040 (Dorsey et al, 2018) • Aging populations, increasing longevity, decreasing smoking rates, and the by- products of industrialization MDS Clinical Diagnostic Criteria for PD Postuma et al 2015 MDS Clinical Diagnostic Criteria for PD Postuma et al 2015 Non-motor Symptoms of PD • Neuropsychiatric symptoms • Autonomic dysfunction • Depression up to 50-70 % • Drooling • Anxiety up to 60 % • Orthostatic hypotension 30–58% • Apathy 60 % • Urinary dysfunction • Psychosis up to 40% • Erectile dysfunction • Impulse control and related disorders • Gastrointestinal dysfunction 25–67% • Dementia, 24% to 40%. • Excessive sweating • Cognitive impairment 20-25% (other • Others than dementia mainly mild cognitive impairment) • Pain 30–85% • Fatigue 50% • Disorders of sleep and wakefulness • Olfactory dysfunction 90% • • Sleep fragmentation and insomnia Ophthalmologic dysfunction • Rapid eye movement sleep behavior disorder 65 % • Excessive daytime sleepiness Pathophysiology of Motor & NMS Neuropathology of PD: Motor Symptoms Two major pathologic processes: (a) premature selective loss of dopamine neurons: 30–70% cell loss ⇢ motor symptoms. (b) the accumulation of Lewy bodies, composed of α-synuclein Poewe, W. -

Abscesses Are a Serious Problem for People Who Shoot Drugs
Where to Get Your Abscess Seen Abscesses are a serious problem for people who shoot drugs. But what the hell are they and where can you go for care? What are abscesses? Abscesses are pockets of bacteria and pus underneath you skin and occasionally in your muscle. Your body creates a wall around the bacteria in order to keep the bacteria from infecting your whole body. Another name for an abscess is a “soft tissues infection”. What are bacteria? Bacteria are microscopic organisms. Bacteria are everywhere in our environment and a few kinds cause infections and disease. The main bacteria that cause abscesses are: staphylococcus (staff-lo-coc-us) aureus (or-e-us). How can you tell when you have an abscess? Because they are pockets of infection abscesses cause swollen lumps under the skin which are often red (or in darker skinned people darker than the surrounding skin) warm to the touch and painful (often VERY painful). What is the worst thing that can happen? The worst thing that can happen with abscesses is that they can burst under your skin and cause a general infection of your whole body or blood. An all over bacterial infection can kill you. Another super bad thing that can happen is a endocarditis, which is an infection of the lining of your heart, and “septic embolism”, which means that a lump of the contaminates in your abscess get loose in your body and lodge in your lungs or brain. Why do abscesses happen? Abscesses are caused when bad bacteria come in to contact with healthy flesh. -

NDA/BLA Multi-Disciplinary Review and Evaluation
NDA/BLA Multi-disciplinary Review and Evaluation NDA 214154 Nextstellis (drospirenone and estetrol tablets) NDA/BLA Multi-Disciplinary Review and Evaluation Application Type NDA Application Number(s) NDA 214154 (IND 110682) Priority or Standard Standard Submit Date(s) April 15, 2020 Received Date(s) April 15, 2020 PDUFA Goal Date April 15, 2021 Division/Office Division of Urology, Obstetrics, and Gynecology (DUOG) / Office of Rare Diseases, Pediatrics, Urologic and Reproductive Medicine (ORPURM) Review Completion Date April 15, 2021 Established/Proper Name drospirenone and estetrol tablets (Proposed) Trade Name Nextstellis Pharmacologic Class Combination hormonal contraceptive Applicant Mayne Pharma LLC Dosage form Tablet Applicant proposed Dosing x Take one tablet by mouth at the same time every day. Regimen x Take tablets in the order directed on the blister pack. Applicant Proposed For use by females of reproductive potential to prevent Indication(s)/Population(s) pregnancy Recommendation on Approval Regulatory Action Recommended For use by females of reproductive potential to prevent Indication(s)/Population(s) pregnancy (if applicable) Recommended Dosing x Take one pink tablet (drospirenone 3 mg, estetrol Regimen anhydrous 14.2 mg) by mouth at the same time every day for 24 days x Take one white inert tablet (placebo) by mouth at the same time every day for 4 days following the pink tablets x Take tablets in the order directed on the blister pack 1 Reference ID: 4778993 NDA/BLA Multi-disciplinary Review and Evaluation NDA 214154 Nextstellis (drospirenone and estetrol tablets) Table of Contents Table of Tables .................................................................................................................... 5 Table of Figures ................................................................................................................... 7 Reviewers of Multi-Disciplinary Review and Evaluation ................................................... -

Cellulitis (You Say, Sell-You-Ly-Tis)
Cellulitis (you say, sell-you-ly-tis) Any area of skin can become infected with cellulitis if the skin is broken, for example from a sore, insect bite, boil, rash, cut, burn or graze. Cellulitis can also infect the flesh under the skin if it is damaged or bruised or if there is poor circulation. Signs your child has cellulitis: The skin will look red, and feel warm and painful to touch. There may be pus or fluid leaking from the skin. The skin may start swelling. The red area keeps growing. Gently mark the edge of the infected red area How is with a pen to see if the red area grows bigger. cellulitis spread? Red lines may appear in the skin spreading out from the centre of the infection. Bad bacteria (germs) gets into broken skin such as a cut or insect bite. What to do Wash your hands before and Cellulitis is a serious infection that needs to after touching the infected area. be treated with antibiotics. Keep your child’s nails short and Go to the doctor if the infected area is clean. painful or bigger than a 10 cent piece. Don’t let your child share Go to the doctor immediately if cellulitis is bath water, towels, sheets and near an eye as this can be very serious. clothes. Make sure your child takes the antibiotics Make sure your child rests every day until they are finished, even if and eats plenty of fruit and the infection seems to have cleared up. The vegetables and drinks plenty of antibiotics need to keep killing the infection water. -

COVID-19 Mrna Pfizer- Biontech Vaccine Analysis Print
COVID-19 mRNA Pfizer- BioNTech Vaccine Analysis Print All UK spontaneous reports received between 9/12/20 and 22/09/21 for mRNA Pfizer/BioNTech vaccine. A report of a suspected ADR to the Yellow Card scheme does not necessarily mean that it was caused by the vaccine, only that the reporter has a suspicion it may have. Underlying or previously undiagnosed illness unrelated to vaccination can also be factors in such reports. The relative number and nature of reports should therefore not be used to compare the safety of the different vaccines. All reports are kept under continual review in order to identify possible new risks. Report Run Date: 24-Sep-2021, Page 1 Case Series Drug Analysis Print Name: COVID-19 mRNA Pfizer- BioNTech vaccine analysis print Report Run Date: 24-Sep-2021 Data Lock Date: 22-Sep-2021 18:30:09 MedDRA Version: MedDRA 24.0 Reaction Name Total Fatal Blood disorders Anaemia deficiencies Anaemia folate deficiency 1 0 Anaemia vitamin B12 deficiency 2 0 Deficiency anaemia 1 0 Iron deficiency anaemia 6 0 Anaemias NEC Anaemia 97 0 Anaemia macrocytic 1 0 Anaemia megaloblastic 1 0 Autoimmune anaemia 2 0 Blood loss anaemia 1 0 Microcytic anaemia 1 0 Anaemias haemolytic NEC Coombs negative haemolytic anaemia 1 0 Haemolytic anaemia 6 0 Anaemias haemolytic immune Autoimmune haemolytic anaemia 9 0 Anaemias haemolytic mechanical factor Microangiopathic haemolytic anaemia 1 0 Bleeding tendencies Haemorrhagic diathesis 1 0 Increased tendency to bruise 35 0 Spontaneous haematoma 2 0 Coagulation factor deficiencies Acquired haemophilia -

Evaluation of Symptom Heterogeneity in Neuropathic Pain Using Assessments of Sensory Functions
Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics Evaluation of Symptom Heterogeneity in Neuropathic Pain Using Assessments of Sensory Functions Kathrin Arning and Ralf Baron Division of Neurological Pain Research and Therapy, Department of Neurology, Christian-Albrechts-Universität Kiel, Germany Summary: Classification of neuropathic pain has been based can be elucidated in each patient. Moreover, in questionnaires on disease entities, anatomical localization, or histological ob- the verbal descriptors can depict the quality and intensity of the servations. Over the past decade, there has been an explosion in individual pain. The approach of classifying and subgrouping our understanding of the basic mechanisms of neuropathic pain. patients with neuropathic pain on the basis of symptoms or The exciting advances in basic science are paralleled by the signs opens up new possibilities for stratifying patients in clin- recognition from clinical investigations that neuropathic pain is ical trials. First, in clinical proof-of-concept trials the study not a monolithic entity, but instead presents as a composite of population can be enriched prospectively on the basis of entry pain and other sensory symptoms. Attempts are under way to criteria defined a priori. This enrichment with patients who supplement the traditional classification with a classification potentially require a specific treatment should increase the like- that links pain and sensory symptoms with neurobiological lihood for positive trial outcomes. Second, in clinical practice it mechanisms. This mechanism- or symptom-based classifica- becomes possible to establish an individualized therapy—that tion takes both negative and positive sensory symptoms into is, to identify the particular patients who require a specific account.