Laboratory Diagnosis of Gonococcal Infections * ALICE REYN, M.D.'
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Abscesses Are a Serious Problem for People Who Shoot Drugs
Where to Get Your Abscess Seen Abscesses are a serious problem for people who shoot drugs. But what the hell are they and where can you go for care? What are abscesses? Abscesses are pockets of bacteria and pus underneath you skin and occasionally in your muscle. Your body creates a wall around the bacteria in order to keep the bacteria from infecting your whole body. Another name for an abscess is a “soft tissues infection”. What are bacteria? Bacteria are microscopic organisms. Bacteria are everywhere in our environment and a few kinds cause infections and disease. The main bacteria that cause abscesses are: staphylococcus (staff-lo-coc-us) aureus (or-e-us). How can you tell when you have an abscess? Because they are pockets of infection abscesses cause swollen lumps under the skin which are often red (or in darker skinned people darker than the surrounding skin) warm to the touch and painful (often VERY painful). What is the worst thing that can happen? The worst thing that can happen with abscesses is that they can burst under your skin and cause a general infection of your whole body or blood. An all over bacterial infection can kill you. Another super bad thing that can happen is a endocarditis, which is an infection of the lining of your heart, and “septic embolism”, which means that a lump of the contaminates in your abscess get loose in your body and lodge in your lungs or brain. Why do abscesses happen? Abscesses are caused when bad bacteria come in to contact with healthy flesh. -

Cellulitis (You Say, Sell-You-Ly-Tis)
Cellulitis (you say, sell-you-ly-tis) Any area of skin can become infected with cellulitis if the skin is broken, for example from a sore, insect bite, boil, rash, cut, burn or graze. Cellulitis can also infect the flesh under the skin if it is damaged or bruised or if there is poor circulation. Signs your child has cellulitis: The skin will look red, and feel warm and painful to touch. There may be pus or fluid leaking from the skin. The skin may start swelling. The red area keeps growing. Gently mark the edge of the infected red area How is with a pen to see if the red area grows bigger. cellulitis spread? Red lines may appear in the skin spreading out from the centre of the infection. Bad bacteria (germs) gets into broken skin such as a cut or insect bite. What to do Wash your hands before and Cellulitis is a serious infection that needs to after touching the infected area. be treated with antibiotics. Keep your child’s nails short and Go to the doctor if the infected area is clean. painful or bigger than a 10 cent piece. Don’t let your child share Go to the doctor immediately if cellulitis is bath water, towels, sheets and near an eye as this can be very serious. clothes. Make sure your child takes the antibiotics Make sure your child rests every day until they are finished, even if and eats plenty of fruit and the infection seems to have cleared up. The vegetables and drinks plenty of antibiotics need to keep killing the infection water. -

Factsheet Boils and Impetigo
FACTSHEET BOILS AND IMPETIGO WHAT IS A BOIL? HOW CAN YOU STOP THE SPREAD OF BOILS A boil is an infection of the skin, usually caused by AND IMPETIGO? Staphylococcus bacteria. Boils are tender, swollen sores, Wash your hands which are full of pus. The tenderness usually goes away, Hand washing is the most important way to prevent the once the boil bursts and the pus and fluid drain. spread of boils and impetigo. Wash all parts of your hands (including between the fingers and under fingernails) WHAT IS IMPETIGO? vigorously with soap and running water for 10-15 seconds. Impetigo is an infection of the skin caused by either Rinse well and dry your hands (with a paper towel if you Staphylococcus or Streptococcus bacteria. The symptoms can). Wash your hands: of impetigo are either small blisters or flat, honey-coloured crusty sores, on the skin. Impetigo is sometimes called • before and after touching or dressing an infected area ‘school sores’. or wound; • after going to the toilet; HOW ARE BOILS AND IMPETIGO TREATED? • after blowing your nose; If you have the symptoms of boils or impetigo: • before handling or eating food; • see your doctor for advice on the treatment of both; • before handling newborn babies; • if sores are small or few, local antiseptic cream and hot • after touching or handling unwashed clothing or linen; compresses may help; • after handling animals or animal waste. • your doctor may prescribe you antibiotic tablets or Cover boils and impetigo ointment. It is important to take the full course of antibiotics. If you don’t, the sores may come back. -

Building Blocks of Clinical Practice Helping Athletic Trainers Build a Strong Foundation
Building Blocks of Clinical Practice Helping Athletic Trainers Build a Strong Foundation Issue #2: Bacterial Infections of the Skin Folliculitis Carbuncles Definition: Definition: • Inflammation of a hair follicle • A complication of folliculitis, a carbuncle is several • Can progress down hair follicle or into multiple furuncles that have merged.merged follicles and result in a furuncle or carbuncle • Carbuncles are readily transmitted by skin-to-skin contact. Often, the direct cause of a carbuncle cannot Causes: be determined.determined • Bacterial or viral infection, chemical irritation • Secondary to skin injury, which introduces bacteria to Causes: the area • Most carbuncles are caused by the bacteria • Shaving hairy areas may facilitate infection staphylococcus aureus. The infection is contagious and • Occlusion of hair-bearing areas may facilitate growth may spread to other areas of the body or other people.people of microbes • Friction Symptoms: • Hyperhidrosis • A carbuncle is a swollen lump or mass under the skin which may be the size of a pea or as larlargege as a golf ball.ball Symptoms: • The carbuncle may be red and irritated and might hurt • Areas are usually non-tender or slightly tender when you touch it.it • Area may itch • Pain gets worse as it fills with pus and dead tissue.tissue • Erythematous perifollicular papules or pustules may • Other signs and symptoms include itching at the site of develop infection, skin inflammation around the wound, general • Grouped lesions ill feeling, fever, or fatigue. Pain improves -

Management of Bacterial Infections of the Hair Follicles – Folliculitis and Furunculosis What Are Bacterial Infections of Hair Follicles? 1
Disclaimer: Treatment of skin diseases, common in developing countries, is often based on the use of simple management schemes which are easy to apply and cheap. The protocols here were devised to provide examples of recognition and treatment schedules which meet these criteria. It is important to recognise that these are seldom supported by a strong evidence base as many of the treatments were devised and assessed many years ago. However we hope that they provide useful examples of treatment protocols that can be applied in different countries using medications that are commonly available. They are broadly based on the evidence presented in the Disease Control Priorities project (www.dcp2.org). Management of bacterial infections of the hair follicles – folliculitis and furunculosis What are bacterial infections of hair follicles? 1. Causative organism: Staphylococcus aureus. 2. When this organism invades the hair follicle it causes one of 3 different clinical patterns of infection: folliculitis, furunculosis ( boils ) or carbuncles: • Folliculitis is a superficial infection of single hair follicles, • Furuncles are deeper infections of the hair follicles • Carbuncles represent the coalescence of a number of furuncles. 3. It is possible for individuals to carry Staphylococcus aureus in the anterior nose, axillae, perineum and on some skin rashes eg eczema, psoriasis Which population is at risk? 1. Folliculitis: Hair-bearing skin and after application of greasy ointments which can cover or occlude the hair follicles. 2. Furuncles (also known as boils): These are often seen in those who carry Staphylococcus (especially in nasal passages), patients with a tendency to develop eczema - rarely in patients with underlying diseases such as conditions that lead to immunosuppression or diabetes. -

How to Treat and Prevent Boils
REGIONAL HEALTH EDUCATION REGIONAL HEALTH EDUCATION How to treat and prevent boils Boils are red, swollen, painful bumps under the skin. Often, they form when a hair follicle gets infected. The infection forms an abscess or pocket of pus, sometimes growing larger than a ping-pong ball. A boil can be extremely painful. Prevention The best ways to prevent boils are to avoid wearing tight clothing and to wash boil-prone areas often with soapy water. These areas include the face, neck, armpits, breasts, groin, and buttocks. To avoid spreading the bacteria that causes a boil to develop, you should: • Wash your hands frequently, especially after touching or treating a boil • Avoid sharing personal items such as razors or towels Home treatment In general, if a boil is not large or excessively painful, home treatment can be effective in resolving the problem. Once a boil develops, resist the urge to squeeze, scratch, drain, or lance it. This can make the infection worse. Instead, gently wash the area well with soap and water twice a day. Dry it well, but don’t rub. You can also apply hot washcloths to the boil 3 or 4 times a day, for 20 minutes or more at a stretch. You might also try a hot water bottle or heating pad applied over a damp towel. These warm compresses can help bring the boil to a head, although the process may take 5 to 7 days. If the boil opens, keep applying the compresses for three more days. An opened boil should be covered with a bandage. -

Incidence of External Ear Canal Folliculitis
Journal of Rawalpindi Medical College (JRMC); 2017;21(1): 75-77 Original Article Incidence of External Ear Canal Folliculitis Mohammad Iqbal1, Kamran Iqbal2,Sahibzada Fawad Khan3,Wasim Ahmad4 1.Department of Otolaryngology, Head & Neck Surgery, Bannu Medical College Bannu;2.Department of ENT, Gomal Medical College D.I.Khan; 3.Department of ENT, Nowshera Medical College;4.Department of Biotechnology, Faculty of Biological Sciences, University of Science & Technology, Bannu Abstract Folliculitis is termed as an inflammation and infection of hair follicles, the minute openings in the skin from Background: To analyze the incidence of External which hair nurtures. Folliculitis is due to an incursion Ear Canal Folliculitis (EECF) in adult population of of bacteria that enter the follicles and cause a bacterial district Bannu. infection.1 Folliculitis is most frequently the outcome Methods: In this descriptive study 100 patients of an infection of bacteria, staphylococcal. This causes with ear ache, presented in four quarters of the year inflammation and a red rash that is jarring and were included. Complaints, and findings on physical scratchy. The rash can happen on the skin or scalp any examination including otoscopy results, diagnosis where. were recorded. Inclusion criteria was adults (18+) The body attempts to combat the bacterial infection by with ear pain as a major complaint. We included the transporting white blood cells to the infected follicles. cases with another major complaint only when it This can end in the formation of pus-filled sores. In was related to the ear pain (e.g. referred pain from some cases folliculitis can lead to the formation of a sinusitis, tonsillitis).Exclusion criteria was antibiotic boil.The inflammation can be either limited to the treatment in the last 10 days was marked as superficial aspect of the follicle with primary exclusion criteria. -

Bacterial Skin Infections
What is Impetigo? • When treated appropriately, impetigo heals over a • Avoid touching the infected areas and prevent others • Impetigo is a superficial bacterial infection of the few days without leaving scars, although there may from touching them too. skin. be temporary redness and darker pigmentation that • Wash hands after touching affected area. • The most common bacteria causing impetigo is may take weeks or months to resolve. • Wash hands before and after applying creams or Staphylococcus aureus, or “Staph aureus”. Less ointments to the affected area. commonly, it can be caused by another bacteria, How is impetigo diagnosed? • Do not share towels or personal items until the Streptococcus. • Impetigo is diagnosed by the typical appearance and infected area is completely healed. Bacterial Skin • Impetigo is common in young children but can symptoms. • Do not let your child return to school or childcare also affect people of other ages, especially those • A skin swab from the affected area may be taken to facilities until the lesions are dried and healed. Infections with skin that is damaged by cuts, insect bites, or identify the bacteria. eczema. • If the infection recurs, a swab from your child’s nose What is erysipelas/cellulitis? • The bacteria can spread by skin-to-skin contact or by to test for the presence of “Staph aureus” may be • Erysipelas and cellulitis are bacterial infections touching contaminated surfaces such as towels and indicated. affecting deeper layers of skin. clothes. • The most common bacteria causing erysipelas or How is impetigo treated? cellulitis is Streptococcus, but other bacteria may be Treatment depends on the severity and extent of the involved, including Staphylococcus aureus. -

THE CEREBROSPINAL FLUID in SYPHILIS.* the Results Presented
THE CEREBROSPINAL FLUID IN SYPHILIS.* BY ARTHUR W. M. ELLIS, M.B., AND HOMER F. SWIFT, M.D. (From the Hospital of The Rockefeller Institute for Medical Research, New York.) The results presented in this communication are based on the examination of 113 cases of syphilis in all stages of the disease, from the secondary eruption to general paresis. During the past ten months every case of syphilis admitted to the hospital has had lumbar puncture performed as part of the routine examination; previous to that time only cases which suggested infection of the nervous system were punctured. TECHNIQUE. Lumbar puncture is ahvays performed with the patient lying on the right side. It has been found that a uniform position for all patients greatly increases the ease of the procedure, and special stress is laid on having the patient properly placed in the beginning. The troublesome headache sometimes seen after lumbar puncture is less frequent with the patient prone, than when the puncture is per- formed with the patient sitting up. The skin and underlying tissues at the site of puncture are infiltrated with a little 2 per cent. novo- caine solution. This makes the procedure much less painful and patients rarely object to repeated punctures. The routine examina- tion of the fluid is confined to four procedures,--estimation of pres- sure, cell count, globulin determination, and Wassermann reaction. Pressure.--The pressure is estimated by allowing the fluid to run into a graduated manometer tube, with a bore three millimeters in diameter, and reading the height to which the fluid rises. -

Laboratory Methods for the Diagnosis of Meningitis Caused by Neisseria Meningitidis, Streptococcus Pneumoniae, and Haemophilus Influenzae WHO Manual, 2Nd Edition
WHO/IVB.11.09 Laboratory Methods for the Diagnosis of Meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae WHO MANUAL, 2ND EDITION Photo: Jon Shadid/UNICEF WHO/IVB.11.09 Laboratory Methods for the Diagnosis of Meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae WHO MANUAL, 2ND EDITION1 1 The first edition has the WHO reference WHO/CDS/CSR/EDC/99.7: Laboratory Methods for the Diagnosis of Meningitis caused by Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae, http://whqlibdoc.who.int/hq/1999/WHO_CDS_CSR_EDC_99.7.pdf © World Health Organization 2011 This document is not a formal publication of the World Health Organization. All rights reserved. This document may, however, be reviewed, abstracted, reproduced and translated, in part or in whole, but not for sale or for use in conjunction with commercial purposes. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. -
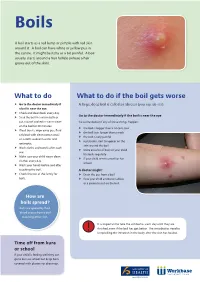
What to Do What to Do If the Boil Gets Worse Go to the Doctor Immediately If a Large, Deep Boil Is Called an Abscess (You Say, Ab-Ses)
Boils A boil starts as a red lump or pimple with red skin around it. A boil can have white or yellow pus in the centre. It might be itchy or a bit painful. A boil usually starts around a hair follicle (where a hair grows out of the skin). What to do What to do if the boil gets worse Go to the doctor immediately if A large, deep boil is called an abscess (you say, ab-ses). a boil is near the eye. Check and clean boils every day. Go to the doctor immediately if the boil is near the eye Soak the boil in a warm bath or put a towel soaked in warm water Go to the doctor if any of these things happen: on the boil for 20 minutes. the boil is bigger than a 10 cent coin If boil bursts, wipe away pus, fluid the boil lasts longer than a week or blood with clean cotton wool the boil is very painful or a cloth soaked in water and red streaks start to appear on the antiseptic. skin around the boil Wash cloths and towels after each there are a lot of boils or your child use . has boils regularly Make sure your child wears clean if your child seems unwell or has clothes every day. a fever Wash your hands before and after touching the boil. A doctor might: Check the rest of the family for Drain the pus from a boil boils. Give your child antibiotic tablets or a cream to put on the boil How are boils spread? Boils are spread by fluid, blood or pus from a boil touching other skin. -

Minor Procedures
MINOR PROCEDURES ELIZABETH BLUNT, RN, PHD, FNP-BC COLLEEN STELLABOTTE, RN, MSN, FNP-BC Today’s Agenda Digital block Subungual hematoma Ingrown toenail management Nail removal Abscess I & D Indication for Digital Blocks •Patients with crush injuries •Need x-rays •Need extensive wound exploration •Tendon injuries requiring evaluation •Multiple lacerations in same digit •Multiple foreign bodies in same digit •Partial or total nail removal Local Anesthetics •Mechanism of action • Infiltrate tissue and diffuse across neural sheaths and membranes • Interfere with neuronal depolarization • Prevent Na influx • No action potential, no nerve impulse •Onset of Action • Technique of injection, concentration, total dose • Knowledge of anatomy is important • Injection between dermis and subcutaneous tissue = immediate anesthesia •Duration of action • Increased with epinephrine • Do not use epinephrine in fingers, toes, nose, penis, ears • Increased with long acting anesthetics such as Bupivicaine and Marcaine Wound Anesthesia GOAL = Reduce Pain and Anxiety • Type of anesthetic used • Needle size 27 or 30-gauge • Inexperienced – start with 25-gauge • 25-gauge or 27-gauge ½-inch needle • pH - buffering • Temperature of solution • Speed and depth of injection Three Major Techniques for Digital Block • Dorsal surface • Indicated when only the nail is involved • Aberrant anatomy may make the technique less than optimal • Web space • Need larger volume of anesthetic • Less control of instillation • Medial/lateral • Focused area of instillation • Volume parameters