Epigenetics in Radiation-Induced Fibrosis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Magazin Wirtschaft
www.stuttgart.ihk.de 07.2013 Stuttgart - Böblingen - Esslingen-Nürtingen - Göppingen - Ludwigsburg - Rems-Murr Magazin Wirtschaft Ein Service der IHK für Unternehmen in der Region Stuttgart Fachkräfte finden zwischen Alb und Gäu Seite 6 wer hat schon ein ganzes kabinett nur für diewirtschaft? Selbstverständlich wir. Damit die Betriebe gute Rahmenbedingungen haben, sind in unseren Gremien ausschließlich Wirtschaftexperten vertreten. www.stuttgart.ihk.de oder Infoline 0711 2005-0 EDITORIAL Handelskriege kennen keine Gewinner Ähnlich dem Frühjahrswetter in diesem weit kommen? China ist die zweitgrößte Wirt- Jahr ziehen nun auch Wolken über dem schaftsmacht der Welt. Daher sollte das Land Parkett des internationalen Handels beginnen, sich an internationale Spielregeln auf. Auslöser ist die vorläufige Erhebung von zu halten. Globalisierung benötigt faire Wett- EU-Strafzöllen auf chinesische Solarmodule. bewerbsbedingungen, an die sich China zu- China kontert mit einem Anti-Dumping-Ver- weilen jedoch nicht hält. Belegte Fälle von fahren gegen europäischen Wein. Immer Wettbewerbsverstößen gibt es genug. mehr Produkte treten in den Fokus der Dum- ping-Diskussion. Vernünftige Handelspolitik Die EU muss schnellstens sieht anders aus. Mangels Deeskalation wird Verhandlungen aufnehmen die Situation für China und Europa immer tassilo zywietz unangenehmer. Ein Handelskrieg droht. Europa darf davor die Augen nicht ver- Geschäftsführer International Globalisierung erfordert jedoch gegensei- schließen. Nachgiebigkeit ist keine Erfolg ver- der IHK Region Stuttgart tige Rücksichtnahme und Handeln im Sinne sprechende Strategie – damit würde die EU der immer stärker miteinander verbundenen erpressbar. Lässt man China gewähren, kann Volkswirtschaften: China und die EU haben die Methode künftig in der Chemiebranche letztes Jahr Waren und Dienstleistungen in und irgendwann im Autobau angewendet Höhe von über 400 Milliarden Euro ausge- werden. -

Zion Lutheran's Surname List
Zion Lutheran Church Records 1861–1961, Belleville, Illinois—surnames of families found in this book. © 2011 St. Clair County Genealogical Society, P.O. Box 431, Belleville, IL 62222-0431. Early records of this church—translated from German—include 4200 baptisms with parents named, 2700 confirmation entries, 1268 marriages, 1360 burials, witnesses and sponsors. A CDRom publication is planned by the SCCGS. Interested in a copy? Contact the Society via one of the website www.stclair-ilgs.org links posted there. _EIMAND ANDRECK BAEHR BARTHELHEIMER BEIRAND BETTERTSCH ABEL ANDREGG BAETTGER BARTLING BEISER BETZ ABENDROTH ANDRES BAEUERLE BARTON BEISSWINGERT BEUDA ABERLE ANDREW BAEUMER BARTS BEITERMANN BEURMANN ABRAHAMSON ANDREWS BAGNAUER BARTTELBORT BEITHAUS BEUSE ACHISON ANDRO BAGNET BARTZ BELCOUR BEUTENBACH ACHS ANDRUSHAT BAGSHAW BATER BELINSKI BEUTNAGEL ACKER ANNA BAHORCE BATMAN BELKER BEVIART ACKERMANN ANSTETTE BAHORICH BATRIE BELKERS BEWART ACOEN ANTEROPP BAHR BATSSTE BELL BEYER ADAMS ANTHES BAIER BATTELBORD BELLEVILLE BEYERLEY ADAMSON ANTHONY BAIL BATTOE BELLOFF BICKEL ADELMANN ANTON BAILEY BAUCHER BELMKE BIEBEL ADEN APEL BAKER BAUER BENDE BIEBER ADKINS APPERSON BALAICH BAUMAN BENDER BIEBN ADLER AREY BALARCH BAUMANN BENDIN BIEDERMANN ADRIAN ARING BALARICK BAUMGARTEN BENDINGER BIEHL AGLES ARL BALDEN BAUMGARTNER BENEDICK BIEHLHORN AGNE ARMANNO BALDWIN BAUMUNCH BENEDICT BIEN AHLEMEYER ARMANNS BALKE BAYER BENING BIERERLY AHLERS ARMBRUST BALL BAZ BENNIKE BIERLENBACH AHLERSMEIER ARMBRUSTER BALLHAUS BEAN BENNING BIERMANN AHLERSMEYER ARMENO BALSEKER -
Marriage Certificates
GROOM LAST NAME GROOM FIRST NAME BRIDE LAST NAME BRIDE FIRST NAME DATE PLACE Abbott Calvin Smerdon Dalkey Irene Mae Davies 8/22/1926 Batavia Abbott George William Winslow Genevieve M. 4/6/1920Alabama Abbotte Consalato Debale Angeline 10/01/192 Batavia Abell John P. Gilfillaus(?) Eleanor Rose 6/4/1928South Byron Abrahamson Henry Paul Fullerton Juanita Blanche 10/1/1931 Batavia Abrams Albert Skye Berusha 4/17/1916Akron, Erie Co. Acheson Harry Queal Margaret Laura 7/21/1933Batavia Acheson Herbert Robert Mcarthy Lydia Elizabeth 8/22/1934 Batavia Acker Clarence Merton Lathrop Fannie Irene 3/23/1929East Bethany Acker George Joseph Fulbrook Dorothy Elizabeth 5/4/1935 Batavia Ackerman Charles Marshall Brumsted Isabel Sara 9/7/1917 Batavia Ackerson Elmer Schwartz Elizabeth M. 2/26/1908Le Roy Ackerson Glen D. Mills Marjorie E. 02/06/1913 Oakfield Ackerson Raymond George Sherman Eleanora E. Amelia 10/25/1927 Batavia Ackert Daniel H. Fisher Catherine M. 08/08/1916 Oakfield Ackley Irving Amos Reid Elizabeth Helen 03/17/1926 Le Roy Acquisto Paul V. Happ Elsie L. 8/27/1925Niagara Falls, Niagara Co. Acton Robert Edward Derr Faith Emma 6/14/1913Brockport, Monroe Co. Adamowicz Ian Kizewicz Joseta 5/14/1917Batavia Adams Charles F. Morton Blanche C. 4/30/1908Le Roy Adams Edward Vice Jane 4/20/1908Batavia Adams Edward Albert Considine Mary 4/6/1920Batavia Adams Elmer Burrows Elsie M. 6/6/1911East Pembroke Adams Frank Leslie Miller Myrtle M. 02/22/1922 Brockport, Monroe Co. Adams George Lester Rebman Florence Evelyn 10/21/1926 Corfu Adams John Benjamin Ford Ada Edith 5/19/1920Batavia Adams Joseph Lawrence Fulton Mary Isabel 5/21/1927Batavia Adams Lawrence Leonard Boyd Amy Lillian 03/02/1918 Le Roy Adams Newton B. -
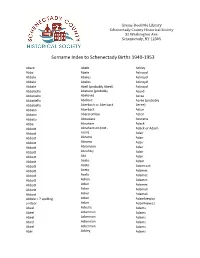
Surname Index to Schenectady Births 1940-1953
Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Abare Abele Ackley Abba Abele Ackroyd Abbale Abeles Ackroyd Abbale Abeles Ackroyd Abbale Abell (probably Abeel) Ackroyd Abbatiello Abelone (probably Acord Abbatiello Abelove) Acree Abbatiello Abelove Acree (probably Abbatiello Aberbach or Aberback Aeree) Abbato Aberback Acton Abbato Abercrombie Acton Abbato Aboudara Acucena Abbe Abraham Adack Abbott Abrahamson (not - Adack or Adach Abbott nson) Adair Abbott Abrams Adair Abbott Abrams Adair Abbott Abramson Adair Abbott Abrofsky Adair Abbott Abt Adair Abbott Aceto Adam Abbott Aceto Adamczak Abbott Aceto Adamec Abbott Aceto Adamec Abbott Acken Adamec Abbott Acker Adamec Abbott Acker Adamek Abbott Acker Adamek Abbzle = ? spelling Acker Adamkiewicz unclear Acker Adamkiewicz Abeel Ackerle Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abeel Ackerman Adams Abel Ackley Adams Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. Schenectady, NY 12305 Surname Index to Schenectady Births 1940-1953 Adams Adamson Ahl Adams Adanti Ahles Adams Addis Ahman Adams Ademec or Adamec Ahnert Adams Adinolfi Ahren Adams Adinolfi Ahren Adams Adinolfi Ahrendtsen Adams Adinolfi Ahrendtsen Adams Adkins Ahrens Adams Adkins Ahrens Adams Adriance Ahrens Adams Adsit Aiken Adams Aeree Aiken Adams Aernecke Ailes = ? Adams Agans Ainsworth Adams Agans Aker (or Aeher = ?) Adams Aganz (Agans ?) Akers Adams Agare or Abare = ? Akerson Adams Agat Akin Adams Agat Akins Adams Agen Akins Adams Aggen Akland Adams Aggen Albanese Adams Aggen Alberding Adams Aggen Albert Adams Agnew Albert Adams Agnew Albert or Alberti Adams Agnew Alberti Adams Agostara Alberti Adams Agostara (not Agostra) Alberts Adamski Agree Albig Adamski Ahave ? = totally Albig Adamson unclear Albohm Adamson Ahern Albohm Adamson Ahl Albohm (not Albolm) Adamson Ahl Albrezzi Grems-Doolittle Library Schenectady County Historical Society 32 Washington Ave. -

Participant List
Participant List 10/20/2019 8:45:44 AM Category First Name Last Name Position Organization Nationality CSO Jillian Abballe UN Advocacy Officer and Anglican Communion United States Head of Office Ramil Abbasov Chariman of the Managing Spektr Socio-Economic Azerbaijan Board Researches and Development Public Union Babak Abbaszadeh President and Chief Toronto Centre for Global Canada Executive Officer Leadership in Financial Supervision Amr Abdallah Director, Gulf Programs Educaiton for Employment - United States EFE HAGAR ABDELRAHM African affairs & SDGs Unit Maat for Peace, Development Egypt AN Manager and Human Rights Abukar Abdi CEO Juba Foundation Kenya Nabil Abdo MENA Senior Policy Oxfam International Lebanon Advisor Mala Abdulaziz Executive director Swift Relief Foundation Nigeria Maryati Abdullah Director/National Publish What You Pay Indonesia Coordinator Indonesia Yussuf Abdullahi Regional Team Lead Pact Kenya Abdulahi Abdulraheem Executive Director Initiative for Sound Education Nigeria Relationship & Health Muttaqa Abdulra'uf Research Fellow International Trade Union Nigeria Confederation (ITUC) Kehinde Abdulsalam Interfaith Minister Strength in Diversity Nigeria Development Centre, Nigeria Kassim Abdulsalam Zonal Coordinator/Field Strength in Diversity Nigeria Executive Development Centre, Nigeria and Farmers Advocacy and Support Initiative in Nig Shahlo Abdunabizoda Director Jahon Tajikistan Shontaye Abegaz Executive Director International Insitute for Human United States Security Subhashini Abeysinghe Research Director Verite -

2017 Annual Report
2017 Annual Report Celebrating 113 Years ~ Serving Sailors, Marines, and their families 2017 OUR MISSION TABLE OF CONTENTS To provide, in partnership with the Navy and Marine Corps, financial, educational and other assistance to Greetings from the Secretary of the Navy ...............3 members of the Naval Service of the United States, their A Message from the Commandant of the eligible family members and survivors when in need; Marine Corps ...........................................................4 and to receive and manage funds to administer these programs. A Message from the Chief of Naval Operations ......5 President’s Year in Review......................................6 VISION Report of the Relief Committee ...............................7 As a non-profit, volunteer service organization, we Report of the Finance Committee............................8 use both financial and non-financial resources to Financial Position and Summary of Operations ......9 identify solutions to meet emerging needs. We help clients improve personal financial skills and encourage Financial Highlights ...............................................10 individual financial responsibility. A Comparison of Financial Assistance to Contributions .........................................................11 GUIDING PRINCIPLES Financial Assistance & Active Duty Fund Drive Results .................................................12 – 13 We provide effective client service in a consistent, compassionate, and non-judgmental manner. Volunteer Recognition ...........................................14 -

A Foundation for the Future
A FOUNDATION FOR THE FUTURE INVESTORS REPORT 2012–13 NORTHWESTERN UNIVERSITY Dear alumni and friends, As much as this is an Investors Report, it is also living proof that a passion for collaboration continues to define the Kellogg community. Your collective support has powered the forward movement of our ambitious strategic plan, fueled development of our cutting-edge curriculum, enabled our global thought leadership, and helped us attract the highest caliber of students and faculty—all key to solidifying our reputation among the world’s elite business schools. This year, you also helped set a new record for alumni support of Kellogg. Our applications and admissions numbers are up dramatically. We have outpaced our peer schools in career placements for new graduates. And we have broken ground on our new global hub. Your unwavering commitment to everything that Kellogg stands for helps make all that possible. Your continuing support keeps us on our trajectory to transform business education and practice to meet the challenges of the new economy. Thank you for investing in Kellogg today and securing the future for generations of courageous leaders to come. All the best, Sally Blount ’92, Dean 4 KELLOGG.NORTHWESTERN.EDU/INVEST contentS 6 Transforming Together 8 Early Investors 10 Kellogg Leadership Circle 13 Kellogg Investors Leaders Partners Innovators Activators Catalysts who gave $1,000 to $2,499 who gave up to $1,000 99 Corporate Affiliates 101 Kellogg Investors by Class Year 1929 1949 1962 1975 1988 2001 1934 1950 1963 1976 1989 2002 -

Surname First Name Categorisation Abadin Jose Luis Silver Abbelen
2018 DRIVERS' CATEGORISATION LIST Updated on 09/07/2018 Drivers in red : revised categorisation Drivers in blue : new categorisation Surname First name Categorisation Abadin Jose Luis Silver Abbelen Klaus Bronze Abbott Hunter Silver Abbott James Silver Abe Kenji Bronze Abelli Julien Silver Abergel Gabriele Bronze Abkhazava Shota Bronze Abra Richard Silver Abreu Attila Gold Abril Vincent Gold Abt Christian Silver Abt Daniel Gold Accary Thomas Silver Acosta Hinojosa Julio Sebastian Silver Adam Jonathan Platinum Adams Rudi Bronze Adorf Dirk Silver Aeberhard Juerg Silver Afanasiev Sergei Silver Agostini Riccardo Gold Aguas Rui Gold Ahlin-Kottulinsky Mikaela Silver Ahrabian Darius Bronze Ajlani Karim Bronze Akata Emin Bronze Aksenov Stanislas Silver Al Faisal Abdulaziz Silver Al Harthy Ahmad Silver Al Masaood Humaid Bronze Al Qubaisi Khaled Bronze Al-Azhari Karim Bronze Alberico Neil Silver Albers Christijan Platinum Albert Michael Silver Albuquerque Filipe Platinum Alder Brian Silver Aleshin Mikhail Platinum Alesi Giuliano Silver Alessi Diego Silver Alexander Iradj Silver Alfaisal Saud Bronze Alguersuari Jaime Platinum Allegretta Vincent Silver Alleman Cyndie Silver Allemann Daniel Bronze Allen James Silver Allgàuer Egon Bronze Allison Austin Bronze Allmendinger AJ Gold Allos Manhal Bronze Almehairi Saeed Silver Almond Michael Silver Almudhaf Khaled Bronze Alon Robert Silver Alonso Fernando Platinum Altenburg Jeff Bronze Altevogt Peter Bronze Al-Thani Abdulrahman Silver Altoè Giacomo Silver Aluko Kolawole Bronze Alvarez Juan Cruz Silver Alzen -

PETITION List 04-30-13 Columns
PETITION TO FREE LYNNE STEWART: SAVE HER LIFE – RELEASE HER NOW! • 1 Signatories as of 04/30/13 Arian A., Brooklyn, New York Ilana Abramovitch, Brooklyn, New York Clare A., Redondo Beach, California Alexis Abrams, Los Angeles, California K. A., Mexico Danielle Abrams, Ann Arbor, Michigan Kassim S. A., Malaysia Danielle Abrams, Brooklyn, New York N. A., Philadelphia, Pennsylvania Geoffrey Abrams, New York, New York Tristan A., Fort Mill, South Carolina Nicholas Abramson, Shady, New York Cory a'Ghobhainn, Los Angeles, California Elizabeth Abrantes, Cambridge, Canada Tajwar Aamir, Lawrenceville, New Jersey Alberto P. Abreus, Cliffside Park, New Jersey Rashid Abass, Malabar, Port Elizabeth, South Salma Abu Ayyash, Boston, Massachusetts Africa Cheryle Abul-Husn, Crown Point, Indiana Jamshed Abbas, Vancouver, Canada Fadia Abulhajj, Minneapolis, Minnesota Mansoor Abbas, Southington, Connecticut Janne Abullarade, Seattle, Washington Andrew Abbey, Pleasanton, California Maher Abunamous, North Bergen, New Jersey Andrea Abbott, Oceanport, New Jersey Meredith Aby, Minneapolis, Minnesota Laura Abbott, Woodstock, New York Alexander Ace, New York, New York Asad Abdallah, Houston, Texas Leela Acharya, Toronto, Canada Samiha Abdeldjebar, Corsham, United Kingdom Dennis Acker, Los Angeles, California Mohammad Abdelhadi, North Bergen, New Jersey Judith Ackerman, New York, New York Abdifatah Abdi, Minneapolis, Minnesota Marilyn Ackerman, Brooklyn, New York Hamdiya Fatimah Abdul-Aleem, Charlotte, North Eddie Acosta, Silver Spring, Maryland Carolina Maria Acosta, -

The Impact of Giving
The impact of giving ANNUAL FUNDRAISING REPORT • 2012–13 Recognising the supporters of Imperial College London 1 AUGUST 2012–31 JULY 2013 From the President & Rector SECURING IMPERIAL’S FUTURE SUCCEss Building on the success of our fundraising One of the highlights of my efforts throughout 2012–13, I’m delighted tenure as President & Rector that we have recently launched the Imperial has been the acquisition 1851 Circle, which celebrates the generosity of 25 acres of land in White of donors who make an annual contribution City last year that will of between £1,000 and £4,999 to the College. soon become home to our Giving at any level is received with genuine new campus — Imperial gratitude; and we would like to particularly West. Imperial West will acknowledge our leadership donors, in create London’s first major recognition of their significant investment in research and translation the College. I extend a very warm welcome to quarter, and enable the all new members and look forward to seeing College to undertake the Imperial 1851 Circle donor pins on display research, translation and Sir Keith O’Nions, President & Rector at College events. commercialisation with The collective support of our individual, corporate and charitable partners — financially and through contributions of time and knowledge — enables Imperial to attract and educate partner organisations on an thousands of students every year. Imperial’s graduation ceremony is held in the iconic Last summer, we welcomed Professor James unprecedented scale for London and the UK. Royal Albert Hall and is a celebration of each and every student’s achievements. -

A3344 Publication Title: Membership Applications to the NS-Frauenshcaft
Publication Number: A3344 Publication Title: Membership Applications to the NS-Frauenshcaft/Deutsches Frauenwerk Date Published: n.d. MEMBERSHIP APPLICATIONS TO THE NS-FRAUENSHCAFT/DEUTSCHES FRAUENWERK Introduction Berlin Document Center (BDC) biographic records include membership information for approximately 3.5 million members of the NS Frauenschaft and Deutsches Frauenwerk (National Socialist Women’s Organization), which directed the activities of various women’s organizations in Nazi Germany. Included among the latter were professional organizations, NSDAP women’s auxiliary formations, and humanitarian and educational groups during the 1931-45 period. As with other ‘front’ organizations, NSDAP membership was not required, so that many individuals documented here are not found elsewhere among BDC biographic collections. The Records are organized in two series, reproduced on 2,418 microfilm rolls. The earliest Frauenschaft organizations came into existence in October 1931 as NSDAP women’s auxiliaries. After Hitler came to power in January 1933, the NS-Frauenschaft served as the means by which existing women’s organizations were brought into line with National Socialist principles. Under the direction of Reichsfauenführerin Gertrud Scholtz-Klink, it specialized in political indoctrination of such varied organizations as the Reichsgemeinschaft Deutscher Hausfauen (Reich Community of German Housewives), Bund Deutscher Ärztinnen (League of German Female Physicians), and others. Its organization paralleled that of the Nazi Party, with subordinate -

Bride Surname Bride First Name Bridegroom Surname Bridegroom First Name Date of Marriage (Berger) Sutter Cath. Kutz Hermann 5 Oc
St. Louis County Library History Genealogy Dept. Index to By name of bride [email protected] | 314-994-3300 ext. 2070 St. Liborius marriages, 1856-1895 Date of Bride Surname Bride First Name Bridegroom Surname Bridegroom First Name Marriage (Berger) Sutter Cath. Kutz Hermann 5 Oct 1893 (Kluempers) Borchers Magdalena Schmidt Franziscus 2 May 1893 Abel Catharina Riesenbeck Hermann 21 Nov 1876 Adam Dorothea Kastien Friedr. 8 Aug 1876 Adel Louisa Bange Josephus 19 Oct 1871 Aggenhwert Catharina Stohlamnn Ernst 20 Jan 1885 Albers Bertha Vetter Lorenz 4 Oct 1888 Albitz Emma Maderer Ignaz 17 Feb 1885 Allers Maria Gesina Kleinegger Franciscus Johannes 19 May 1859 Altebockwinkel Gertrudis Huhmann Wilhelmus 17 Oct 1857 Altemeier Louisa Fischer Otto 15 Jan 1873 Altemeier Anna Läger Johannes Gerhard. 31 Oct 1866 Altengreve Catharina Bothe Georgius 18 Apr 1871 Applebaum Francisca Guenther Carl 15 May 1884 Applebaum Maria Kussmann Friedrich 10 May 1883 Applebaum Clara Wigge Georg 15 May 1888 Aselage Maria Hoormann Hermannus 21 Jun 1859 Atzot Gertrudis Hei Martinus 5 Sept 1858 Austermann Maria Peitz Georg 17 Nov 1874 Austermann Theresia Zurline Friedrich 12 Feb 1890 Aut Maria Elisabeth Butterwegge Theodorus 4 Nov 1858 Bain Maria Gertrud Bathe Franz 9 May 1876 Banscher Thecla Abele Joseph 4 Oct 1881 Bartholomae Francisca Schrew Hermann 3 Feb 1874 Bartmeier Elisabeth Flies Casparus 28 Jul 1864 Bartscher Catharina Dickhaus Hermannus 2 Apr 1861 Bauer Margaretha Volmerd Hermann 6 Apr 1891 Baumgartner Amalia Stock Jacob 18 Oct 1894 Beck Maria Ditmeier Georg 8 May 1892 Beck Margaretha Sievers Joseph 22 Nov 1892 1 St. Louis County Library History Genealogy Dept.