Chemical Society Reviews REVIEW
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)
Western University Scholarship@Western Electronic Thesis and Dissertation Repository 8-8-2013 12:00 AM Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling) Rana Sabouni The University of Western Ontario Supervisor Prof. Sohrab Rohani The University of Western Ontario Graduate Program in Chemical and Biochemical Engineering A thesis submitted in partial fulfillment of the equirr ements for the degree in Doctor of Philosophy © Rana Sabouni 2013 Follow this and additional works at: https://ir.lib.uwo.ca/etd Part of the Other Chemical Engineering Commons Recommended Citation Sabouni, Rana, "Carbon Dioxide Adsorption by Metal Organic Frameworks (Synthesis, Testing and Modeling)" (2013). Electronic Thesis and Dissertation Repository. 1472. https://ir.lib.uwo.ca/etd/1472 This Dissertation/Thesis is brought to you for free and open access by Scholarship@Western. It has been accepted for inclusion in Electronic Thesis and Dissertation Repository by an authorized administrator of Scholarship@Western. For more information, please contact [email protected]. i CARBON DIOXIDE ADSORPTION BY METAL ORGANIC FRAMEWORKS (SYNTHESIS, TESTING AND MODELING) (Thesis format: Integrated Article) by Rana Sabouni Graduate Program in Chemical and Biochemical Engineering A thesis submitted in partial fulfilment of the requirements for the degree of Doctor of Philosophy The School of Graduate and Postdoctoral Studies The University of Western Ontario London, Ontario, Canada Rana Sabouni 2013 ABSTRACT It is essential to capture carbon dioxide from flue gas because it is considered one of the main causes of global warming. Several materials and various methods have been reported for the CO2 capturing including adsorption onto zeolites, porous membranes, and absorption in amine solutions. -

The Royal Society of Chemistry Turns Its Focus on Researchers with Better Search and Measurement Tools
The Royal Society of Chemistry turns its focus on researchers with better search and measurement tools The Royal Society of Chemistry offers a publishing platform providing access to over a million chemical science articles, book chapters and abstracts. Like many publishers of high quality peer-reviewed content, they are under pressure from their community to innovate quickly and harness digital technology in new ways that add value, simplicity and easier access to the research workflow. About Will Russell is responsible for some of the new technical developments • pubs.rsc.org at the Royal Society of Chemistry. “Although we do a lot of in-house • rsc.org development, we need to understand where developments can be • Location: Cambridge UK with improved by working with partners,” he says. “I really believe in the additional editorial teams in Beijing, benefit of strategic technology partnerships with an external partner. China, Bangalore India and There is the speed of getting a key utility to the market and this offers Washington D.C. USA us a tremendous business advantage.” • Scientific publisher of high-impact journals and books “We have journals going back to 1841,” he says. “We started migrating People print content online in the late 1990s. Our biggest challenge now is how • Will Russell we will deliver content in the future in the most useful way for the Business Relationship Manager researcher.” Goals Will pinpoints a way forward. “There are new opportunities presented • Embrace new technology to remain by open science and alternative metrics, and increasing importance competitive against innovative attached to data and open data,” he says. -

Winter for the Membership of the American Crystallographic Association, P.O
AMERICAN CRYSTALLOGRAPHIC ASSOCIATION NEWSLETTER Number 4 Winter 2004 ACA 2005 Transactions Symposium New Horizons in Structure Based Drug Discovery Table of Contents / President's Column Winter 2004 Table of Contents President's Column Presidentʼs Column ........................................................... 1-2 The fall ACA Council Guest Editoral: .................................................................2-3 meeting took place in early 2004 ACA Election Results ................................................ 4 November. At this time, News from Canada / Position Available .............................. 6 Council made a few deci- sions, based upon input ACA Committee Report / Web Watch ................................ 8 from the membership. First ACA 2004 Chicago .............................................9-29, 38-40 and foremost, many will Workshop Reports ...................................................... 9-12 be pleased to know that a Travel Award Winners / Commercial Exhibitors ...... 14-23 satisfactory venue for the McPherson Fankuchen Address ................................38-40 2006 summer meeting was News of Crystallographers ...........................................30-37 found. The meeting will be Awards: Janssen/Aminoff/Perutz ..............................30-33 held at the Sheraton Waikiki Obituaries: Blow/Alexander/McMurdie .................... 33-37 Hotel in Honolulu, July 22-27, 2005. Council is ACA Summer Schools / 2005 Etter Award ..................42-44 particularly appreciative of Database Update: -

Chemistry Subject Ejournal Packages
Chemistry subject eJournal packages Subject Included journals No. of journals Analyst; Biomaterials Science; Food & Function; Journal of Materials Chemistry B; Lab on a Chip; Metallomics; Molecular Omics; Biological chemistry Molecular Systems Design & Engineering; Photochemical & Photobiological Sciences; Toxicology Research 10 Catalysis Science & Technology; Dalton Transactions; Energy & Environmental Science; Green Chemistry; Organic & Biomolecular Chemistry; Catalysis science Photochemical & Photobiological Sciences; Physical Chemistry Chemical Physics; Reaction Chemistry & Engineering 8 Lab on a Chip; MedChemComm; Metallomics; Molecular Omics; Natural Product Reports; Organic & Biomolecular Chemistry; Photochemical Biochemistry & Photobiological Sciences; Toxicology Research 8 CrystEngComm; Energy & Environmental Science; Green Chemistry; Journal of Materials Chemistry A; Molecular Systems Design & Energy Engineering; Physical Chemistry Chemical Physics; Journal of Materials Chemistry 7 Energy & Environmental Science; Environmental Science: Nano; Environmental Science: Processes & Impacts; Environmental Science: Environmental Science Water Research & Technology; Green Chemistry; Journal of Materials Chemistry A & C; Photochemical & Photobiological Sciences; Reaction 8 Chemistry & Engineering Food science Analyst; Analytical Methods; Food & Function; Lap on a Chip 4 Catalysis Science & Technology; CrystEngComm; Dalton Transactions; Inorganic Chemistry Frontiers; Metallomics; Photochemical & Inorganic chemistry Photobiological Sciences; -

Data Curation Issues in the Chemical Sciences
ARTICLE EXCERPTED FROM: INFORMATION STANDARDS QUARTERLY FALL 2013 | VOL 25 | ISSUE 3 | ISSN 1041-0031 TOPIC DATA CURATION DATA CURATION ISSUES IN THE CHEMICAL SCIENCES DATA CURATION IN THE OPENAIRE SCHOLARLY COMMUNICATION INFRASTRUCTURE PRESERVING THE GREY LITERATURE EXPLOSION: PDF/A AND THE DIGITAL ARCHIVE ENSURING THE LONG TERM IMPACT OF EARTH SCIENCE DATA THROUGH DATA CURATION AND PRESERVATION COLIN L. BIRD, CERYS WILLOUGHBY, SIMON J. COLES, & JEREMY G. FREY DATA CURATION ISSUES IN THE CHEMICAL SCIENCES All science is strongly dependent on preserving, maintaining, and adding value to the research record, including the data, both raw and derived, generated during the scientific process. This statement leads naturally to the assertion that all science is strongly dependent on curation.[1] Chemistry is no exception, and given the significance of chemical data to many other disciplines, we assert that curation should be a fundamental aspect of the research practice in the chemical sciences. In this article we investigate the extent to which chemists do actually respect the importance of curation in their day-to-day activities in the laboratory or, nowadays, frequently at the computer. » CONTINUED » 6 FE CONTINUED » RESEARCH LIFECYCLE NOTIONAL TIMELINE CURATION PRESERVATION DISCOVERY ACCESS PROVENANCE Figure 1: The concepts of preservation, curation, provenance, discovery, and access in the context of the research lifecycle Reused with permission[3] Several of us have examined the origins and evolution of that group related items, provide context, and facilitate the scientific record keeping in our recent review of laboratory reuse of specified research outputs. [2] a notebooks in the digital era. We compare the electronic Zeng and Qin[4] present examples taken from a math laboratory notebook (ELN) with the traditional paper notebook, dictionary and an educational library system to “demonstrate noting that paper still has some advantages for maintaining that metadata is capable of performing the following tasks: a “journal” of research activities. -

New Journal and Database Subscriptions – 2012 -2013
NEW JOURNAL AND DATABASE SUBSCRIPTIONS – 2012 -2013 New Journals Afterall: A Journal of Art, Context and Enquiry American Biology Teacher American Journal of Bioethics American Political Thought Annals of Tourism Research Art Documentation Biodiversity and Conservation Biomaterials Science BioScience Boom: A Journal of California California Archaeology California Management Review Catalysis Science & Technology Chemical Hazards in Industry China Journal Classical Antiquity Classical Philology Crime and Justice Critical Review of International Social and Political Philosophy Education in Chemistry Educational Technology Research Development Elephant Ethics Federal Sentencing Reporter Food & Function Frankie Gastronomica: The Journal of Food and Culture Haaretz Historical Studies in the Natural Sciences HOPOS: The Journal of the International Society for the History of Philosophy of Science Huntington Library Quarterly Indian Country Today Indonesia Journal Information, Communication & Society Innovation Policy and the Economy Integrative Biology Issues in Environmental Science and Technology Journal of Applied Remote Sensing Journal of Digital Media Management Journal of Empirical Research on Human Research Ethics Journal of Environmental Studies and Sciences Journal of Human Capital Journal of Labor Economics Journal of Leisure Research Journal of Micro/Nanolithography, MEMS, and MOEMS Journal of Modern History Journal of Nanophotonics Journal of North African Studies Journal of Palestine Studies Journal of Photonics for Energy Journal -

RSC Gold 2015 Flyer.Pdf
RSC Gold Want access to full content from the world’s leading chemistry society? Including regular new material and an Archive dating back to 1841? Caltech’s RSC Gold Plus voucher codes to publish package subscription has been a very Open Access (OA) free of charge? welcome development ... I am very appreciative of the RSC Gold is the Royal Society of Chemistry’s general excellence of articles in the RSC premium package comprising 41 international research journals, evidenced by strong journals, literature updating services and impact factors and magazines that will meet the needs of all your increases in local download statistics. end-users. And the accompanying Gold for Gold Dana L. Roth OA voucher codes ensure maximum visibility for Chemistry Librarian your institution’s quality research. Caltech, USA Take a look inside to see exactly what you get www.rsc.org/gold RSC Gold includes a wealth of quality RSC journal, database and magazine content that is all available online. Journals Natural Product Reports Analyst New Journal of Chemistry Analytical Methods Organic & Biomolecular Chemistry Biomaterials Science Photochemical & Photobiological Sciences Catalysis Science & Technology Physical Chemistry Chemical Physics (PCCP) Chemical Communications Polymer Chemistry Chemical Science* RSC Advances Chemical Society Reviews Soft Matter CrystEngComm Toxicology Research Dalton Transactions Energy & Environmental Science B a c k fi l e Environmental Science: Nano** RSC Journals Archive 1841-2007 lease Environmental Science: Processes & Impacts -

Publishing Update 2020
Publishing update 2020 We are an international, not-for-profit organisation connecting chemical scientists with each other, with other scientists, and with society as a whole. Founded in 1841 and based in London, UK, we have a high profile reputation as an international publisher with 46 peer-reviewed journals, more than 1,500 print books and a collection of online databases and literature updating services. We are here to give every mind in the chemical sciences the information and tools to try something new – to help everyone feel confident enough to challenge the accepted and get a step closer to answering fundamental questions. Registered charity number: 207890 New from our journals Journal family updates Horizons The Horizons journals publish short reports of They are focal points for research that pushes exceptional high quality and innovation. the boundaries and challenges current thinking. Volume 5 Volume 2 Number 3 Number 1 May 2018 January 2017 Materials Pages 311-580 Nanoscale Pages 1-66 Horizons Horizons The home for rapid reports of exceptional significance in nanoscience and nanotechnology rsc.li/materials-horizons rsc.li/nanoscale-horizons IMPACT IMPACT FACTOR FACTOR ISSN 2051-6347 ISSN 2055-6756 COMMUNICATION PAPER Blaise L. Tardy, Orlando J. Rojas et al. Xiao Cheng Zeng, Hui Zhao et al. Biofabrication of multifunctional nanocellulosic 3D structures: Type-I van der Waals heterostructure formed by MoS2 and a facile and customizable route 14.356* ReS2 monolayers 9.095* Materials Horizons Nanoscale Horizons Materials Horizons publishes exceptionally high quality, Nanoscale Horizons is the home for exceptionally high quality, innovative materials science, placing emphasis on original innovative nanoscience & nanotechnology. -

Publishing Price List 2016
Publishing Price List 2016 Royal Society of Chemistry Collections for 2016 RSC GOLD INCLUDES: Key Royal Society of Chemistry online Price on application ONLINE ONLY† journal, database and magazine content, plus a EMAIL [email protected] book series. or contact your Account Manager PRICES JOURNALS ARCHIVE ONLINE ONLY† RSC Journals Archive Outright Purchase (1841 – 2007) • £41,097 • $72,615 RSC Journals Archive Outright Purchase (2005 – 2007) • £4,867 • $8,271 RSC Journals Archive Lease (1841 – 2007) • £7,408 • $13,133 PRICES RSC Journals Archive Hosting Fee • £819 • $1,340 THE HISTORICAL COLLECTION INCLUDES: Price on application ONLINE ONLY† • Society Publications (1949 – 2012) EMAIL [email protected] • Society Minutes (1841 – 1966) or contact your Account Manager • Historical Papers (1505 – 1991) PRICES CORE CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Dalton Transactions • Journal of Materials Chemistry A, B & C • New Journal of Chemistry PRINT & ONLINE† ONLINE ONLY† • Organic & Biomolecular Chemistry • • • Physical Chemistry Chemical Physics £19,685 £18,701 • RSC Advances (online only) • $36,814 • $34,973 PRICES GENERAL CHEMISTRY COLLECTION INCLUDES: • Chemical Communications • Chemical Society Reviews PRINT & ONLINE† ONLINE ONLY† • Chemistry World • £8,360 • £7,942 • New Journal of Chemistry PRICES • $13,685 • $13,244 • RSC Advances (online only) ANALYTICAL SCIENCE COLLECTION INCLUDES: • Analyst • Analytical Abstracts (online only) • Analytical Methods PRINT & ONLINE† ONLINE ONLY† • Environmental Science: Processes & Impacts • -

Journal Automatic Opt-In Unknown When Available After Acceptance
Journal Automatic Opt-In Unknown When Available After Acceptance American Chemical Society Accounts of Chemical Research X 30 minutes - 24 hours ACS Applied Materials & Interfaces X 30 minutes - 24 hours ACS Biomaterials Science & Engineering X 30 minutes - 24 hours ACS Catalysis X 30 minutes - 24 hours ACS Chemical Biology X 30 minutes - 24 hours ACS Chemical Neuroscience X 30 minutes - 24 hours ACS Combinatorial Science X 30 minutes - 24 hours ACS Infectious Diseases X 30 minutes - 24 hours ACS Macro Letters X 30 minutes - 24 hours ACS Medicinal Chemistry Letters X 30 minutes - 24 hours ACS Nano X 30 minutes - 24 hours ACS Photonics X 30 minutes - 24 hours ACS Sustainable Chemistry & Engineering X 30 minutes - 24 hours ACS Symposium Series X 30 minutes - 24 hours ACS Synthetic Biology X 30 minutes - 24 hours Advances in Chemistry X 30 minutes - 24 hours Analytical Chemistry X 30 minutes - 24 hours Biochemistry X 30 minutes - 24 hours Bioconjugate Chemistry X 30 minutes - 24 hours Biomacromolecules X 30 minutes - 24 hours Biotechnology Progress X 30 minutes - 24 hours Chemical & Engineering New Archives X 30 minutes - 24 hours C&EN Online X 30 minutes - 24 hours Chemical Research in Toxicology X 30 minutes - 24 hours Chemical Reviews X 30 minutes - 24 hours Chemistry of Materials X 30 minutes - 24 hours Inorganic Chemistry X 30 minutes - 24 hours Journal of the American Chemical Society X 30 minutes - 24 hours Journal of Chemical & Engineering Data X 30 minutes - 24 hours Journal of Chemical Information & Modeling X 30 minutes - 24 hours -

GUT RSC Journals List
SCHEDULE A Publisher Content Section A Customer has access to the electronic versions of the following journals via an External route: Access Post - Copyright Journals E-ISSN years cancellation Owner* during Term access Analyst 1364-5528 2008-2018 2012-2018 RSC Analytical Methods 1 1759-9679 2009-2018 2012-2018 RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2008-2013 2012-2013 RSC B 1460 4779 2008-2013 2012-2013 RSC C 1460-4787 2008-2013 2012-2013 RSC Biomaterials Science 1 2047-4849 2013-2018 2016-2018 RSC Catalysis Science & Technology 1 2044-4761 2011-2018 2013-2018 RSC Chemical Communications 1364-548X 2008-2018 2012-2018 RSC Chemical Science 1, 2 2041-6539 2010-2014 2012-2014 RSC Chemical Society Reviews 1460-4744 2008-2018 2012-2018 RSC Chemistry World 1749-5318 2012-2016 2012-2016 RSC CrystEngComm 1466-8033 2008-2018 2012-2018 RSC Dalton Transactions 1477-9234 2008-2018 2012-2018 RSC Education in Chemistry 1749-5326 2012-2016 2012-2016 RSC Energy & Environmental Science 1 1754-5706 2008-2018 2012-2018 RSC Environmental Science: Nano 1 2051-8161 2014-2018 2016-2018 RSC Environmental Science: Processes & Impacts including 2050-7895 2013-2018 2013-2018 RSC Journal of Environmental Monitoring (1464-0333) 2008-2012 2012 Environmental Science: Water Research & Technology 1 2053-1419 2015-2018 2017-2018 RSC Faraday Discussions 1364-5498 2008-2018 2012-2018 RSC Food & Function 1 2042-650X 2010-2018 2012-2018 RSC Green Chemistry 1463-9270 2008-2018 2012-2018 RSC Inorganic Chemistry Frontiers 1 2052-1553 2014-2018 2017-2018 -
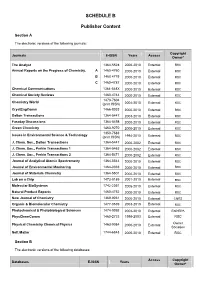
SCHEDULE B Publisher Content
SCHEDULE B Publisher Content Section A The electronic versions of the following journals: Copyright Journals E-ISSN Years Access Owner* The Analyst 1364-5528 2000-2010 External RSC Annual Reports on the Progress of Chemistry, A 1460-4760 2000-2010 External RSC B 1460 4779 2000-2010 External RSC C 1460-4787 2000-2010 External RSC Chemical Communications 1364-548X 2000-2010 External RSC Chemical Society Reviews 1460-4744 2000-2010 External RSC 1473-7604 Chemistry World (print ISSN) 2004-2010 External RSC CrystEngComm 1466-8033 2000-2010 External RSC Dalton Transactions 1364-5447 2003-2010 External RSC Faraday Discussions 1364-5498 2000-2010 External RSC Green Chemistry 1463-9270 2000-2010 External RSC 1350-7583 Issues in Environmental Science & Technology (print ISSN) 1994-2010 External RSC J. Chem. Soc., Dalton Transactions 1364-5447 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 1 1364-5463 2000-2002 External RSC J. Chem. Soc., Perkin Transactions 2 1364-5471 2000-2002 External RSC Journal of Analytical Atomic Spectrometry 1364-5544 2000-2010 External RSC Journal of Environmental Monitoring 1464-0333 2000-2010 External RSC Journal of Materials Chemistry 1364-5501 2000-2010 External RSC Lab on a Chip 1473-0189 2001-2010 External RSC Molecular BioSystems 1742-2051 2005-2010 External RSC Natural Product Reports 1460-4752 2000-2010 External RSC New Journal of Chemistry 1369-9261 2000-2010 External CNRS Organic & Biomolecular Chemistry 1477-0539 2003-2010 External RSC Photochemical & Photobiological Sciences 1474-9092 2002-2010