Comparing Mechanical Mastication
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Natural Heritage Program List of Rare Plant Species of North Carolina 2016
Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Revised February 24, 2017 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2016 Compiled by Laura Gadd Robinson, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1651 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. -

Barcoding the Asteraceae of Tennessee, Tribe Coreopsideae
Schilling, E.E., N. Mattson, and A. Floden. 2014. Barcoding the Asteraceae of Tennessee, tribe Coreopsideae. Phytoneuron 2014-101: 1–6. Published 20 October 2014. ISSN 2153 733X BARCODING THE ASTERACEAE OF TENNESSEE, TRIBE COREOPSIDEAE EDWARD E. SCHILLING, NICHOLAS MATTSON, AARON FLODEN Herbarium TENN Department of Ecology & Evolutionary Biology University of Tennessee Knoxville, Tennessee 37996 [email protected]; [email protected] ABSTRACT Results from barcoding studies of tribe Coreopsideae for the Tennessee flora using the nuclear ribosomal ITS marker are presented and include the first complete reports for 2 of the 20 species of the tribe that occur in the state, as well as updated reports for several others. Sequence data from the ITS region separate most of the species of Bidens in Tennessee from one another, but species of Coreopsis, especially those of sect. Coreopsis, have ITS sequences that are identical (or nearly so) to at least one congener. Comparisons of sequence data to GenBank records are complicated by apparent inaccuracies of older sequences as well as potentially misidentified samples. Broad survey of C. lanceolata from across its range showed little variability, but the ITS sequence of a morphologically distinct sample from a Florida limestone glade area was distinct in lacking a length polymorphism that was present in other samples. Tribe Coreopsideae is part of the Heliantheae alliance and earlier was often included in an expanded Heliantheae (Anderberg et al. 2007) in which it was usually treated as a subtribe (Crawford et al. 2009). The tribe shows a small burst of diversity in the southeastern USA involving Bidens and Coreopsis sect. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2012
Natural Heritage Program List of Rare Plant Species of North Carolina 2012 Edited by Laura E. Gadd, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program Office of Conservation, Planning, and Community Affairs N.C. Department of Environment and Natural Resources 1601 MSC, Raleigh, NC 27699-1601 Natural Heritage Program List of Rare Plant Species of North Carolina 2012 Edited by Laura E. Gadd, Botanist John T. Finnegan, Information Systems Manager North Carolina Natural Heritage Program Office of Conservation, Planning, and Community Affairs N.C. Department of Environment and Natural Resources 1601 MSC, Raleigh, NC 27699-1601 www.ncnhp.org NATURAL HERITAGE PROGRAM LIST OF THE RARE PLANTS OF NORTH CAROLINA 2012 Edition Edited by Laura E. Gadd, Botanist and John Finnegan, Information Systems Manager North Carolina Natural Heritage Program, Office of Conservation, Planning, and Community Affairs Department of Environment and Natural Resources, 1601 MSC, Raleigh, NC 27699-1601 www.ncnhp.org Table of Contents LIST FORMAT ......................................................................................................................................................................... 3 NORTH CAROLINA RARE PLANT LIST ......................................................................................................................... 10 NORTH CAROLINA PLANT WATCH LIST ..................................................................................................................... 71 Watch Category -

Addendum to the Guide to the Natural Communities of the Delaware Estuary
ADDENDUM TO THE UIDE TO THE ATURAL OMMUNITIES G N C OF THE DELAWARE ESTUARY SEPTEMBER0 2009 Citation: Largay, E. and L. Sneddon. 2009. Addendum to the Guide to the Ecological Systems and Vegetation Communities of the Delaware Estuary. NatureServe. Arlington, Virginia. Partnership for the Delaware Estuary, Report #09-XX. 112 pp. PDE Report No. 09-XX Copyright © 2009 NatureServe COVER PHOTOS Top L: Overwash Dunes, photo from Delaware Natural Heritage Program Top R: Coastal Plain Muck Pondshore, photo by Kathleen Strakosch Walz, New Jersey Natural Heritage Program Bottom L: Dry Oak Hickory Forest, photo by Tony Davis, Pennsylvania Natural Heritage Program Bottom R: Inland Dune and Ridge Forest/Woodland, Kathleen Strakosch Walz, New Jersey Natural Heritage Program ADDENDUM TO THE GUIDE TO THE NATURAL COMMUNITIES OF THE DELAWARE ESTUARY Ery Largay Lesley Sneddon September 2009 Acknowledgements: This work was made possible through funding from the Delaware Estuary Program (EPA 320 Funding). Kristin Snow and Mary Russo from NatureServe provided essential data management services to develop this report and report format. Robert Coxe and Bill McAvoy from the Delaware Natural Heritage Program, Kathleen Strakosch Walz from the New Jersey Natural Heritage Program, Tony Davis from the Pennsylvania Natural Heritage Program, Linda Kelly and Karl Anderson, independent botanists, provided ecological expertise, energy and insight. Mark Anderson and Charles Ferree from The Nature Conservancy developed ecological systems maps to accompany this work. Danielle Kreeger, Laura Whalen, and Martha-Maxwell Doyle from the Partnership for the Delaware Estuary provided support and guidance throughout this project. We thank everyone who helped us with this effort. -

Mädchenaugen
Mädchenaugen Die Mädchenaugen (Coreopsis), auch Schöngesicht genannt, sind eine Pflanzengattung innerhalb der Familie Mädchenaugen der Korbblütler (Asteraceae). Nach dem aktuellen Umfang der Gattung kommen alle Arten nur in der Neuen Welt vor. Einige Sorten werden oft als Zierpflanzen kultiviert. Inhaltsverzeichnis Beschreibung Erscheinungsbild und Blätter Blütenstände und Blüten Früchte Chromosomensätze Coreopsis lanceolata, Zuchtform Systematik und Verbreitung Nutzung Systematik Quellen Euasteriden II Einzelnachweise Ordnung: Asternartige (Asterales) Weblinks Familie: Korbblütler (Asteraceae) Unterfamilie: Asteroideae Beschreibung Tribus: Coreopsideae Gattung: Mädchenaugen Wissenschaftlicher Name Coreopsis L. Erscheinungsbild und Blätter Bei Coreopsis-Arten handelt es sich um einjährige oder ausdauernde krautige Pflanzen, seltener auch um Halbsträucher oder um Sträucher. Die meisten Arten erreichen Wuchshöhen von Sektion Gyrophyllum: Quirlblättriges Mädchenauge (Coreopsis verticillata) Illustration des Hohen 10 bis 80 Zentimetern, mit fein fiederteiligen Laubblättern Mädchenauges (Coreopsis tripteris) manche Arten erreichen Wuchshöhen von bis zu 2 Metern oder auch höher. Viele Arten bilden Rhizome oder die Sprossbasis ist verdickt, wenige der Arten (Coreopsis auriculata) können sich mit unter- oder oberirdischen Ausläufern ausbreiten. Bei den meisten Arten wird je Exemplar nur ein selbständig aufrechter Stängel gebildet, die mehr oder weniger auf ihrer gesamten Länge oder erst im oberen Bereich verzweigt sind.[1] Die Laubblätter können -
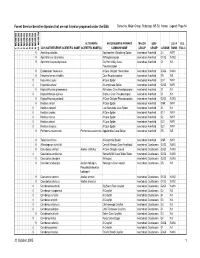
Sensitive Species That Are Not Listed Or Proposed Under the ESA Sorted By: Major Group, Subgroup, NS Sci
Forest Service Sensitive Species that are not listed or proposed under the ESA Sorted by: Major Group, Subgroup, NS Sci. Name; Legend: Page 94 REGION 10 REGION 1 REGION 2 REGION 3 REGION 4 REGION 5 REGION 6 REGION 8 REGION 9 ALTERNATE NATURESERVE PRIMARY MAJOR SUB- U.S. N U.S. 2005 NATURESERVE SCIENTIFIC NAME SCIENTIFIC NAME(S) COMMON NAME GROUP GROUP G RANK RANK ESA C 9 Anahita punctulata Southeastern Wandering Spider Invertebrate Arachnid G4 NNR 9 Apochthonius indianensis A Pseudoscorpion Invertebrate Arachnid G1G2 N1N2 9 Apochthonius paucispinosus Dry Fork Valley Cave Invertebrate Arachnid G1 N1 Pseudoscorpion 9 Erebomaster flavescens A Cave Obligate Harvestman Invertebrate Arachnid G3G4 N3N4 9 Hesperochernes mirabilis Cave Psuedoscorpion Invertebrate Arachnid G5 N5 8 Hypochilus coylei A Cave Spider Invertebrate Arachnid G3? NNR 8 Hypochilus sheari A Lampshade Spider Invertebrate Arachnid G2G3 NNR 9 Kleptochthonius griseomanus An Indiana Cave Pseudoscorpion Invertebrate Arachnid G1 N1 8 Kleptochthonius orpheus Orpheus Cave Pseudoscorpion Invertebrate Arachnid G1 N1 9 Kleptochthonius packardi A Cave Obligate Pseudoscorpion Invertebrate Arachnid G2G3 N2N3 9 Nesticus carteri A Cave Spider Invertebrate Arachnid GNR NNR 8 Nesticus cooperi Lost Nantahala Cave Spider Invertebrate Arachnid G1 N1 8 Nesticus crosbyi A Cave Spider Invertebrate Arachnid G1? NNR 8 Nesticus mimus A Cave Spider Invertebrate Arachnid G2 NNR 8 Nesticus sheari A Cave Spider Invertebrate Arachnid G2? NNR 8 Nesticus silvanus A Cave Spider Invertebrate Arachnid G2? NNR -

Natural Heritage Program List of Rare Plant Species of North Carolina 2018 Revised October 19, 2018
Natural Heritage Program List of Rare Plant Species of North Carolina 2018 Revised October 19, 2018 Compiled by Laura Gadd Robinson, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org STATE OF NORTH CAROLINA (Wataug>f Wnke8 /Madison V" Burke Y H Buncombe >laywoodl Swain f/~~ ?uthertor< /Graham, —~J—\Jo< Polk Lenoii TEonsylvonw^/V- ^ Macon V \ Cherokey-^"^ / /Cloy Union I Anson iPhmonf Ouptln Scotlar Ons low Robeson / Blodon Ponder Columbus / New>,arrfver Brunewlck Natural Heritage Program List of Rare Plant Species of North Carolina 2018 Compiled by Laura Gadd Robinson, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org This list is dynamic and is revised frequently as new data become available. New species are added to the list, and others are dropped from the list as appropriate. The list is published every two years. Further information may be obtained by contacting the North Carolina Natural Heritage Program, Department of Natural and Cultural Resources, 1651 MSC, Raleigh, NC 27699-1651; by contacting the North Carolina Wildlife Resources Commission, 1701 MSC, Raleigh, NC 27699- 1701; or by contacting the North Carolina Plant Conservation Program, Department of Agriculture and Consumer Services, 1060 MSC, Raleigh, NC 27699-1060. Additional information on rare species, as well as a digital version of this list, can be obtained from the Natural Heritage Program’s website at www.ncnhp.org. Cover Photo of Allium keeverae (Keever’s Onion) by David Campbell. TABLE OF CONTENTS INTRODUCTION ................................................................................................................. -

100 Years of Change in the Flora of the Carolinas
DICOTYLEDONS DICOTYLEDONS ACANTHACEAE Durande 1762 (Acanthus Family) A family of about 230 genera and about 3450 species, herbs, shrubs, vines, and trees, largely tropical. References: Wasshausen (1998); Long (1970); McDade & Moody (1999). 1 Leaves in a basal rosette (sometimes with smaller leaves on a scape). 2 Leaves glabrate, to 22 cm long and 8 cm wide; corolla 0.8-1.3 cm long; capsule 8-10 mm long; stamens 2; [of moist to wet swamps] .........................................................................................................................................................Elytraria 2 Leaves pubescent, to 10 cm long and 3 cm wide; corolla 1.8-4 cm long; capsule 9-18 mm long; stamens 4; [of dry upland pinelands]. 3 Leaves 2-10 cm long, 1-3 cm wide; corolla 3-4 cm long; calyx lobes 15-30 mm long; capsule 12-18 mm long........ .............................................................................................................................................................Ruellia ciliosa 3 Leaves 1.5-2.5 cm long, 0.7-0.8 cm wide; corolla ca. 2 cm long; calyx lobes 6-9 mm long; capsule ca. 10 mm long ............................................................................................................................................................... Stenandrium 1 Leaves cauline. 4 Stamens 2; corolla distinctly 2-lipped (except with 4 nearly equal lobes in Yeatesia). 5 Bracts and bractlets inconspicuous, 2-5 mm long, linear or triangular; stem subterete or obscurely 4-angled ........... ........................................................................................................................................................................Justicia -
The Vascular Plants Collected by Mark Catesby in South Carolina: Combining the Sloane and Oxford Herbaria
McMillan, P.D. and A.H. Blackwell. 2013. The vascular plants collected by Mark Catesby in South Carolina: Combining the Sloane and Oxford herbaria. Phytoneuron 2013-73: 1–32. Published 27 September 2013. ISSN 2153 733X THE VASCULAR PLANTS COLLECTED BY MARK CATESBY IN SOUTH CAROLINA: COMBINING THE SLOANE AND OXFORD HERBARIA PATRICK D. MCMILLAN School of Agriculture, Forestry, and the Environment Clemson University Clemson, South Carolina 29634 AMY HACKNEY BLACKWELL Department of Biology Furman University Greenville, South Carolina 29613 and South Carolina Botanical Garden Clemson, South Carolina 29634 ABSTRACT We provide a list of all vascular plant specimens collected in the Carolinas by Mark Catesby that are housed in the historic herbaria at Oxford University and the Sloane Herbarium. The identifications along with notes on the significance of selected specimens are presented. This paper continues our work with Catesby’s collections that we began with his specimens in the Sloane Herbarium at the Natural History Museum, London. The availability of high-quality digital images published on the Oxford Herbarium’s website has facilitated our examination of these specimens. The collections themselves shed light on the nature of the flora of the Carolinas before European settlement, including the native ranges of several problematic taxa. The presence of a number of taxa known to be introduced to the Americas indicates that these introductions must have occurred prior to the 1720s. KEY WORDS: Catesby, Sloane Herbarium, herbarium, historic botany, ecology, South Carolina, digital imaging Mark Catesby, born in England in 1682 or 1683, devoted most of his adult life to studying the natural history of southeastern North America and the Caribbean. -

Natural Heritage Program List of Rare Plant Species of North Carolina 2021
Natural Heritage Program List of Rare Plant Species of North Carolina 2021 Compiled by Brenda L. Wichmann, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org C ur Alleghany rit Ashe Northampton Gates C uc Surry am k Stokes P d Rockingham Caswell Person Vance Warren a e P s n Hertford e qu Chowan r Granville q ot ui a Mountains Watauga Halifax m nk an Wilkes Yadkin s Mitchell Avery Forsyth Orange Guilford Franklin Bertie Alamance Durham Nash Yancey Alexander Madison Caldwell Davie Edgecombe Washington Tyrrell Iredell Martin Dare Burke Davidson Wake McDowell Randolph Chatham Wilson Buncombe Catawba Rowan Beaufort Haywood Pitt Swain Hyde Lee Lincoln Greene Rutherford Johnston Graham Henderson Jackson Cabarrus Montgomery Harnett Cleveland Wayne Polk Gaston Stanly Cherokee Macon Transylvania Lenoir Mecklenburg Moore Clay Pamlico Hoke Union d Cumberland Jones Anson on Sampson hm Duplin ic Craven Piedmont R nd tla Onslow Carteret co S Robeson Bladen Pender Sandhills Columbus New Hanover Tidewater Coastal Plain Brunswick THE COUNTIES AND PHYSIOGRAPHIC PROVINCES OF NORTH CAROLINA Natural Heritage Program List of Rare Plant Species of North Carolina 2021 Compiled by Brenda L. Wichmann, Botanist North Carolina Natural Heritage Program N.C. Department of Natural and Cultural Resources Raleigh, NC 27699-1601 www.ncnhp.org This list is dynamic and is revised every other year as new data become available. New species are added to the list, and others are dropped from the list as appropriate. Further information may be obtained by contacting the North Carolina Natural Heritage Program, Department of Natural and Cultural Resources, 1651 MSC, Raleigh, NC 27699-1651; by contacting the North Carolina Wildlife Resources Commission, 1701 MSC, Raleigh, NC 27699-1701; or by contacting the North Carolina Plant Conservation Program, Department of Agriculture and Consumer Services, 1060 MSC, Raleigh, NC 27699-1060. -

DCR Rare Plant List
COMMONWEALTH of VIRGINIA Natural Heritage Resources of Virginia: Rare Plants November 2016 Compiled by: John F. Townsend, Botanist VIRGINIA DEPARTMENT OF CONSERVATION AND RECREATION DIVISION OF NATURAL HERITAGE 600 EAST MAIN STREET, 24TH FLOOR RICHMOND, VIRGINIA 23219 (804) 786-7951 Cover illustrations (l. to r.) of Swamp Pink (Helonias bullata), dwarf burhead (Echinodorus tenellus), and small whorled pogonia (Isotria medeoloides) by Megan Rollins This report should be cited as: Townsend, John F. 2016. Natural Heritage Resources of Virginia: Rare Plants. Natural Heritage Technical Report 16-18. Virginia Department of Conservation and Recreation, Division of Natural Heritage, Richmond, Virginia. Unpublished report. November 2016. 59 pages plus appendices . INTRODUCTION The Virginia Department of Conservation and Recreation's Division of Natural Heritage (DCR-DNH) was established to protect Virginia's Natural Heritage Resources. These Resources are defined in the Virginia Natural Area Preserves Act of 1989 (Section 10.1-209 through 217, Code of Virginia), as the habitat of rare, threatened, and endangered plant and animal species; exemplary natural communities, habitats, and ecosystems; and other natural features of the Commonwealth. DCR-DNH is the state's only comprehensive program for conservation of our natural heritage and includes an intensive statewide biological inventory, field surveys, electronic and manual database management, environmental review capabilities, and natural area protection and stewardship. Through such a comprehensive operation, the Division identifies Natural Heritage Resources which are in need of conservation attention while creating an efficient means of evaluating the impacts of economic growth. To achieve this protection, DCR-DNH maintains lists of the most significant elements of our natural diversity. -

Epidemiology and Biology of Powdery Mildews and Their Host Plants
Epidemiology and biology of powdery mildews and their host plants Michael Bradshaw A dissertation Submitted in partial fulfillment of the Requirements for the degree of Doctor of Philosophy University of Washington 2020 Reading Committee: Patrick Tobin, Chair Marianne Elliott Soo-Hyung Kim Caroline Strömberg Program Authorized to Offer Degree: Environmental and Forest Sciences © Copyright 2020 Michael Bradshaw University of Washington Abstract Epidemiology and biology of powdery mildews and their host plants Michael Bradshaw Chair of the Supervisory Committee: Professor Patrick Tobin School of Environmental and Forest Science Powdery mildew is one of the most prevalent plant pathogens in the Pacific Northwest with over 150 different species infecting over 1000 plants. The hot, dry summers and wet, mild winters in this region are optimal for its colonization and spread. Sequencing herbarium specimens for plant pathogens, including powdery mildews, can be challenging but useful in addressing fundamental ecological, epidemiological, and phylogenetic questions in plant-pathogen interactions. In my dissertation, I reviewed the taxonomy and phylogeny of powdery mildews and developed a new sequencing protocol for sequencing herbarium specimens. Using this new sequencing protocol, I conducted a world-wide phylogenetic and taxonomic analysis on powdery mildews on Viburnum, in which I described two new species, E. viburniphila sp. nov and E. pseudoviburni sp. nov, and reduced E. hedwigii to synonymy with E. viburni; and genetically ascertained the origin and timing of an introduced plant pathogen of which Acer macrophyllum (bigleaf maple) is highly susceptible. Additionally, I evaluated 126 plant species within Asteraceae to measure the role of host plant morphological traits and evolutionary history on their suitability and susceptibility to the powdery mildew, Golovinomyces latisporus, and observed that phylogenetic structure, and not plant morphology, is the most consistent predictor of host susceptibility to pathogens.