Corporate Profile
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nilotinib in Patients with Chronic Myeloid Leukemia: STAT2 Trial in Japan
Haematologica HAEMATOL/2018/194894 Version 3 Haematologica HAEMATOL/2018/194894 Version 3 Treatment-free remission after two-year consolidation therapy with nilotinib in patients with chronic myeloid leukemia: STAT2 trial in Japan Naoto Takahashi, Kaichi Nishiwaki, Chiaki Nakaseko, Nobuyuki Aotsuka, Koji Sano, Chikako Ohwada, Jun Kuroki, Hideo Kimura, Michihide Tokuhira, Kinuko Mitani, Kazuhisa Fujikawa, Osamu Iwase, Kohshi Ohishi, Fumihiko Kimura, Tetsuya Fukuda, Sakae Tanosaki, Saori Takahashi, Yoshihiro Kameoka, Hiroyoshi Nishikawa, and Hisashi Wakita Disclosures: 1. This study was supported by research funding from Novartis Pharmaceuticals to N.T. 2. N.T reports grants from Novartis Pharmaceuticals, during the conduct of the study; grants and personal fees from Novartis Pharmaceuticals, grants and personal fees from Otsuka, grants and personal fees from Pfizer, personal fees from Bristol-Myers Squibb, outside the submitted work; K.N reports grants from Zenyaku Kogyo Company, Limited, grants from Chugai Pharmaceutical, grants from Novartis Pharma K.K., grants from Kyowa Hakko Kirin Co, Ltd, grants from Nippon Shinyaku Co, Ltd, outside the submitted work; C.N reports personal fees from Novartis, grants and personal fees from Bristol-Myers Squibb, grants and personal fees from Pfizer, grants and personal fees from Takeda pharmaceuticals, grants and personal fees from Kyowa Hakko Kirin, grants and personal fees from Otsuka Pharmaceutical, grants and personal fees from Ono Pharmaceutical, grants and personal fees from Chugai Pharmaceutical, grants and personal fees from Asahi Kasei Pharma, grants and personal fees from Shionogi, personal fees from Shire, personal fees from Jannsen, personal fees from Celgene, outside the submitted work; M.T. reports personal fees from Bristol-Myers Squib, personal fees from Pfizer, outside the submitted work; K.M reports grants from Kyowa Hakko Kirin Co. -

NISSHIN SEIFUN GROUP INC. a Message from the Management
Delivering Good Health and Reliability To Shareholders Business Report 2004 (April 1, 2003 to March 31, 2004) NISSHIN SEIFUN GROUP INC. A Message from the Management CONTENTS A Message from the Management .............1 Interview with the Chairman and the President ....................................3 Feature: Redistributing Profits to Shareholders.......................................7 Feature: Food Product Safety Initiatives ....8 Topics......................................................9 Outline of Nisshin Seifun Group ...............11 Review of Operations..............................13 Osamu Shoda Hiroshi Hasegawa New Products.........................................16 Chairman President Consolidated Financial Highlights ..............17 Forecast for Consolidated Business Performance...........................17 Consolidated Financial Statements..........19 Non-Consolidated Financial Statements...20 Stock Information...................................21 Corporate Data / Investor Information .....22 * The financial data in this report are prepared from the financial statements issued for domestic reporting purpose in accordance with the provisions set forth in the Japanese Securities and Exchange Law and accounting principles generally accepted in Japan. 1 We are pleased to report to you that on a consolidated basis we “Delivering Good Health and Reliability,” in order to further expand achieved our highest levels ever for both net sales and ordinary our operations. In so doing, we will work to fully maximize the income. Net -

Factset-Top Ten-0521.Xlsm
Pax International Sustainable Economy Fund USD 7/31/2021 Port. Ending Market Value Portfolio Weight ASML Holding NV 34,391,879.94 4.3 Roche Holding Ltd 28,162,840.25 3.5 Novo Nordisk A/S Class B 17,719,993.74 2.2 SAP SE 17,154,858.23 2.1 AstraZeneca PLC 15,759,939.73 2.0 Unilever PLC 13,234,315.16 1.7 Commonwealth Bank of Australia 13,046,820.57 1.6 L'Oreal SA 10,415,009.32 1.3 Schneider Electric SE 10,269,506.68 1.3 GlaxoSmithKline plc 9,942,271.59 1.2 Allianz SE 9,890,811.85 1.2 Hong Kong Exchanges & Clearing Ltd. 9,477,680.83 1.2 Lonza Group AG 9,369,993.95 1.2 RELX PLC 9,269,729.12 1.2 BNP Paribas SA Class A 8,824,299.39 1.1 Takeda Pharmaceutical Co. Ltd. 8,557,780.88 1.1 Air Liquide SA 8,445,618.28 1.1 KDDI Corporation 7,560,223.63 0.9 Recruit Holdings Co., Ltd. 7,424,282.72 0.9 HOYA CORPORATION 7,295,471.27 0.9 ABB Ltd. 7,293,350.84 0.9 BASF SE 7,257,816.71 0.9 Tokyo Electron Ltd. 7,049,583.59 0.9 Munich Reinsurance Company 7,019,776.96 0.9 ASSA ABLOY AB Class B 6,982,707.69 0.9 Vestas Wind Systems A/S 6,965,518.08 0.9 Merck KGaA 6,868,081.50 0.9 Iberdrola SA 6,581,084.07 0.8 Compagnie Generale des Etablissements Michelin SCA 6,555,056.14 0.8 Straumann Holding AG 6,480,282.66 0.8 Atlas Copco AB Class B 6,194,910.19 0.8 Deutsche Boerse AG 6,186,305.10 0.8 UPM-Kymmene Oyj 5,956,283.07 0.7 Deutsche Post AG 5,851,177.11 0.7 Enel SpA 5,808,234.13 0.7 AXA SA 5,790,969.55 0.7 Nintendo Co., Ltd. -

Research and Development in Breast Ultrasound E
E. Ueno, T. Shiina, M. Kubota, K. Sawai (Eds.) Research and Development in Breast Ultrasound E. Ueno, T. Shiina M. Kubota, K. Sawai (Eds.) Research and Development in Breast Ultrasound With 150 Figures, Including 37 in Color 1 3 Ei Ueno, M.D., Ph.D. Associate Professor, Department of Breast-Thyroid-Endocrine Surgery Institute of Clinical Medicine, University of Tsukuba 1-1-1 Tennodai, Tsukuba, Ibaraki 304-8573, Japan Tsuyoshi Shiina, Ph.D. Professor, Institute of Information Sciences and Electronics, University of Tsukuba 1-1-1 Tennodai, Tsukuba, Ibaraki 304-8573, Japan Mitsuhiro Kubota, M.D. Director of Yamachika Memorial Hospital 3-19-14 Koyawata, Odawara, Kanagawa 256-0815, Japan Kiyoshi Sawai, M.D. Associate Professor, Department of Endocrine & Breast Surgery Kyoto Prefectural University of Medicine 465 Kajii-cho, Kawaramachi-Hirokoji, Kamigyo-ku, Kyoto 602-0841, Japan Cover Illustration: Madoka Momota Library of Congress Control Number: 2004116202 ISBN 4-431-40277-2 Springer-Verlag Tokyo Berlin Heidelberg New York This work is subject to copyright. All rights are reserved, whether the whole or part of the material is concerned, specifically the rights of translation, reprinting, reuse of illustrations, recitation, broad- casting, reproduction on microfilms or in other ways, and storage in data banks. The use of registered names, trademarks, etc. in this publication does not imply, even in the absence of a specific statement, that such names are exempt from the relevant protective laws and regulations and therefore free for general use. Product liability: The publisher can give no guarantee for information about drug dosage and appli- cation thereof contained in this book. -

Putnam Panagora Market Neutral Fund Q3 Portfolio Holdings
Putnam PanAgora Market Neutral Fund The fund's portfolio 5/31/20 (Unaudited) INVESTMENT COMPANIES (46.1%)(a) Shares Value Morgan Stanley Emerging Markets Domestic Debt Fund, Inc. 640 $3,635 State Street Institutional U.S. Government Money Market Fund 3,939,067 3,939,067 Total investment companies (cost $3,943,561) $3,942,702 UNITS (11.0%)(a) Units Value Acamar Partners Acquisition Corp.(NON) 419 $4,291 Alussa Energy Acquisition Corp. (Cayman Islands)(NON) 856 8,483 Amplitude Healthcare Acquisition Corp.(NON) 2,947 29,529 B. Riley Principal Merger Corp. II(NON) 2,620 26,174 CC Neuberger Principal Holdings I(NON) 2,652 27,024 Chardan Healthcare Acquisition 2 Corp.(NON) 2,652 26,493 CHP Merger Corp.(NON) 2,747 27,745 CIIG Merger Corp.(NON) 4,529 45,335 Collective Growth Corp.(NON) 2,803 27,890 DFP Healthcare Acquisitions Corp.(NON) 2,866 28,746 dMY Technology Group, Inc.(NON) 2,885 29,196 East Stone Acquisition Corp.(NON) 4,230 42,089 FinServ Acquisition Corp.(NON) 831 8,194 Foley Trasimene Acquisition Corp.(NON) 2,626 26,917 Fortress Value Acquisition Corp.(NON) 2,652 26,547 Galileo Acquisition Corp.(NON) 888 8,827 GigCapital3, Inc.(NON) 2,833 28,160 Gores Holdings IV, Inc.(NON) 1,306 13,844 Greenrose Acquisition Corp.(NON) 3,350 32,931 GX Acquisition Corp.(NON) 417 4,233 Healthcare Merger Corp.(NON) 2,705 28,105 InterPrivate Acquisition Corp.(NON) 2,918 29,180 Jaws Acquisition Corp.(NON) 2,620 27,038 Juniper Industrial Holdings, Inc.(NON) 841 8,418 Landcadia Holdings II, Inc.(NON) 1,165 12,174 LGL Systems Acquisition Corp.(NON) 2,568 25,629 Lifesci Acquisition Corp.(NON) 2,866 29,806 LIV Capital Acquisition Corp. -
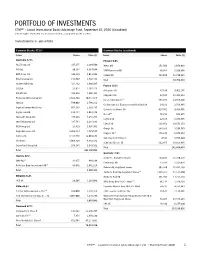
Portfolio of Investments
PORTFOLIO OF INVESTMENTS CTIVP® – Lazard International Equity Advantage Fund, September 30, 2020 (Unaudited) (Percentages represent value of investments compared to net assets) Investments in securities Common Stocks 97.6% Common Stocks (continued) Issuer Shares Value ($) Issuer Shares Value ($) Australia 6.9% Finland 1.0% AGL Energy Ltd. 437,255 4,269,500 Metso OYJ 153,708 2,078,669 ASX Ltd. 80,181 4,687,834 UPM-Kymmene OYJ 36,364 1,106,808 BHP Group Ltd. 349,229 9,021,842 Valmet OYJ 469,080 11,570,861 Breville Group Ltd. 153,867 2,792,438 Total 14,756,338 Charter Hall Group 424,482 3,808,865 France 9.5% CSL Ltd. 21,611 4,464,114 Air Liquide SA 47,014 7,452,175 Data#3 Ltd. 392,648 1,866,463 Capgemini SE 88,945 11,411,232 Fortescue Metals Group Ltd. 2,622,808 30,812,817 Cie de Saint-Gobain(a) 595,105 24,927,266 IGO Ltd. 596,008 1,796,212 Cie Generale des Etablissements Michelin CSA 24,191 2,596,845 Ingenia Communities Group 665,283 2,191,435 Electricite de France SA 417,761 4,413,001 Kogan.com Ltd. 138,444 2,021,176 Elis SA(a) 76,713 968,415 Netwealth Group Ltd. 477,201 5,254,788 Legrand SA 22,398 1,783,985 Omni Bridgeway Ltd. 435,744 1,234,193 L’Oreal SA 119,452 38,873,153 REA Group Ltd. 23,810 1,895,961 Orange SA 298,281 3,106,763 Regis Resources Ltd. -

Chemical Sector Research Analysts SECTOR REVIEW
24 January 2014 Asia Pacific/Japan Equity Research Major Chemicals (Chemicals/Textiles (Japan)) / MARKET WEIGHT Chemical sector Research Analysts SECTOR REVIEW Masami Sawato 81 3 4550 9729 [email protected] Keyword: Innovation Maiko Saito Investment strategy 81 3 4550 9936 [email protected] ■ In 2014, we believe "green innovations" and "life innovations" are key to the longer term growth of chemical makers. ■ Green innovation: In the environment/energy field, solar cells and rechargeable lithium-ion batteries (LiB) should attract greater attention, while lightweight carbon fiber composite materials (CFRP) and their contribution to improving the fuel efficiency of automobiles and aircraft become increasingly important to efforts to reduce energy consumption and CO2 emissions. We believe companies to watch include Toray Industries (3402), Teijin (3401), Mitsubishi Chemical Holdings (4188), Kuraray (4023), Hitachi Chemical (4217), and Ube Industries (4208). ■ Life innovation: For their prospective innovations that could promote greater use of generics and help lower the cost of pharmaceuticals, we are focusing on Nippon Kayaku (4272), a major domestic maker of generic anti- cancer drugs and biosimilars, and Denki Kagaku Kogyo (4061), which we expect to expand its diagnostic reagent business. In addition, we look for Asahi Kasei (3407) to expand its pharmaceuticals business and, over the medium term, its home dialysis business. We think JSR (4185) is interesting for the medium-term growth potential of its drug discovery support business. Finally, Sanyo Chemical Industries (4471) and Nippon Shokubai (4114), two producers of super absorbent polymers (SAP) used in absorbent materials, should benefit from the global growth in demand that we expect for disposable diapers. -
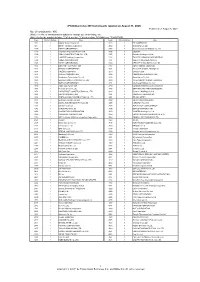
JPX-Nikkei Index 400 Constituents (Applied on August 31, 2021) Published on August 6, 2021 No
JPX-Nikkei Index 400 Constituents (applied on August 31, 2021) Published on August 6, 2021 No. of constituents : 400 (Note) The No. of constituents is subject to change due to de-listing. etc. (Note) As for the market division, "1"=1st section, "2"=2nd section, "M"=Mothers, "J"=JASDAQ. Code Market Divison Issue Code Market Divison Issue 1332 1 Nippon Suisan Kaisha,Ltd. 3048 1 BIC CAMERA INC. 1417 1 MIRAIT Holdings Corporation 3064 1 MonotaRO Co.,Ltd. 1605 1 INPEX CORPORATION 3088 1 Matsumotokiyoshi Holdings Co.,Ltd. 1719 1 HAZAMA ANDO CORPORATION 3092 1 ZOZO,Inc. 1720 1 TOKYU CONSTRUCTION CO., LTD. 3107 1 Daiwabo Holdings Co.,Ltd. 1721 1 COMSYS Holdings Corporation 3116 1 TOYOTA BOSHOKU CORPORATION 1766 1 TOKEN CORPORATION 3141 1 WELCIA HOLDINGS CO.,LTD. 1801 1 TAISEI CORPORATION 3148 1 CREATE SD HOLDINGS CO.,LTD. 1802 1 OBAYASHI CORPORATION 3167 1 TOKAI Holdings Corporation 1803 1 SHIMIZU CORPORATION 3231 1 Nomura Real Estate Holdings,Inc. 1808 1 HASEKO Corporation 3244 1 Samty Co.,Ltd. 1812 1 KAJIMA CORPORATION 3254 1 PRESSANCE CORPORATION 1820 1 Nishimatsu Construction Co.,Ltd. 3288 1 Open House Co.,Ltd. 1821 1 Sumitomo Mitsui Construction Co., Ltd. 3289 1 Tokyu Fudosan Holdings Corporation 1824 1 MAEDA CORPORATION 3291 1 Iida Group Holdings Co.,Ltd. 1860 1 TODA CORPORATION 3349 1 COSMOS Pharmaceutical Corporation 1861 1 Kumagai Gumi Co.,Ltd. 3360 1 SHIP HEALTHCARE HOLDINGS,INC. 1878 1 DAITO TRUST CONSTRUCTION CO.,LTD. 3382 1 Seven & I Holdings Co.,Ltd. 1881 1 NIPPO CORPORATION 3391 1 TSURUHA HOLDINGS INC. 1893 1 PENTA-OCEAN CONSTRUCTION CO.,LTD. -

Convocation Notice of the 170 Ordinary General
This document has been translated from a part of the Japanese original for reference purposes only. In the event of any discrepancy between this translated document and the Japanese original, the original shall prevail. The Company assumes no responsibility for this translation or for direct, indirect or any other forms of damages arising from the translation. (Securities Code: 2002) June 4, 2014 To Those Shareholders with Voting Rights Hiroshi Oeda Director and President Nisshin Seifun Group Inc. 25, Kanda-Nishiki-cho 1-chome, Chiyoda-ku, Tokyo JAPAN CONVOCATION NOTICE OF THE 170th ORDINARY GENERAL MEETING OF SHAREHOLDERS You are cordially invited to attend the 170th Ordinary General Meeting of Shareholders of Nisshin Seifun Group Inc. (the “Company”). The meeting will be held as described below. If you are unable to attend the meeting, you can exercise your voting rights by one of the following methods. Please review the “Reference Documents for the General Meeting of Shareholders,” and exercise your voting rights by 7:00 p.m. (JST), Wednesday, June 25, 2014. [Voting in Writing (by Postal Mail)] Please indicate your vote for or against each of the proposals on the enclosed Voting Rights Exercise Form, and return the form by no later than the aforementioned deadline for the exercise of voting rights. [Voting Electronically (via the Internet)] Please refer to the enclosed “Exercising your Voting Rights via the Internet,” and vote for or against each of the proposals at the voting rights exercise website (http://www.web54.net) by no later than the aforementioned deadline for the exercise of voting rights. -

Securities Report 163Rd Fiscal Term (April 1, 2006 to March 31, 2007)
Disclaimer: This is a Japanese-English translation of the securities report published in Japanese that provides detailed financial statements and other information about the company. The figures contained herein are unaudited, and only the Japanese version can be assumed official. This document does not constitute any guarantee, and the company will not compensate any losses and/or damage stemming from actions taken based on these statements. In the case of any discrepancy between the Japanese and English versions, the Japanese version is assumed correct. 163rd Fiscal Term (April 1, 2006 to March 31, 2007) Securities Report Nisshin Seifun Group Inc. Contents Page Report Data 2 Part A: Company Information 3 [1] Company Overview 3 (1) Principal Business Performance Indicators 3 (2) History 5 (3) Business Overview 6 (4) Subsidiaries and Affiliates 8 (5) Employees 9 [2] Review of Operations & Financial Position 10 (1) Review of Operations 10 (2) Status of Production, Orders Received & Sales 12 (3) Prospective Challenges 13 (4) Business Risks 18 (5) Legal & Contractual Matters 21 (6) Research and Development 22 (7) Analysis of Financial Position & Performance 23 [3] Facilities & Capital Expenditures 27 (1) Capital Expenditures 27 (2) Principal Facilities 28 (3) Facility Construction & Disposal Plans 30 [4] Other Matters Related to Nisshin Seifun Group Inc. 31 (1) Share-related Matters 31 (2) Acquisitions of Treasury Stock 48 (3) Dividend Policy 49 (4) Share Price Movements 49 (5) Directors & Auditors 50 (6) Corporate Governance 53 [5] Financial Accounts 58 (1) Consolidated Financial Statements 59 (2) Non-consolidated Financial Statements 92 [6] Stock-related Administration 119 [7] Corporate Reference Data 121 (1) Information on Parent Company of Nisshin Seifun Group Inc. -

TOBAM Maximum Diversification All World Developed Ex North America USD
TOBAM Maximum Diversification All World Developed ex North America USD 31/12/2019 Instrument Weight BP PLC 0.10% IDEMITSU KOSAN CO LTD 0.21% INPEX HOLDINGS INC 0.07% JX HOLDINGS INC 0.09% NESTE OIL OYJ 1.16% OMV AG 0.08% SANTOS LTD 0.02% SBM OFFSHORE NV 0.05% TGS NOPEC GEOPHYSICAL CO ASA 0.02% VOPAK 0.02% WOOD GROUP (JOHN) PLC 0.02% AIR LIQUIDE 0.23% AIR WATER INC 0.02% AKZO NOBEL 0.12% ALUMINA LTD 0.03% AMCOR PLC-CDI 0.08% AVON RESOURCES LTD 0.53% BORAL LTD 0.02% CHR HANSEN HOLDING A/S 0.08% DAICEL CHEMICAL INDUSTRIES 0.02% DOWA HOLDINGS CO LTD 0.01% EMS-CHEMIE HOLDING AG-REG 0.03% FLETCHER BUILDING LTD 0.02% FORTESCUE METALS GROUP LTD 0.60% GIVAUDAN-REG 0.16% HITACHI CHEMICAL CO LTD 0.03% HUHTAMAKI OYJ 0.03% ISRAEL CHEMICALS LTD 0.02% JAMES HARDIE INDUSTRIES-CDI 0.07% JFE HOLDINGS INC 0.02% KANSAI PAINT CO LTD 0.03% KURARAY CO LTD 0.03% MITSUBISHI MATERIALS CORP 0.02% NEWCREST MINING LTD 1.35% TOBAM Maximum Diversification All World Developed ex North America USD 31/12/2019 Instrument Weight NIPPON PAINT CO LTD 0.05% NIPPON PAPER INDUSTRIES CO L 0.04% NIPPON SHOKUBAI CO LTD 0.01% NISSAN CHEMICAL INDUSTRIES 0.04% NOF CORP 0.02% NORTHERN STAR RESOURCES LTD 0.66% NOVOZYMES A/S-B SHARES 0.07% OJI PAPER CO LTD 0.03% ORICA LTD 0.02% ORORA LTD 0.02% SARACEN MINERAL HOLDINGS LTD 0.32% SMURFIT KAPPA GROUP PLC 0.04% SYMRISE AG 0.04% TAIHEIYO CEMENT CORP 0.02% TAIYO NIPPON SANSO CORP 0.02% TEIJIN LTD 0.02% THYSSENKRUPP AG 0.04% TORAY INDUSTRIES INC 0.02% WIENERBERGER AG 0.02% ADP 0.04% AENA SA 0.09% ALFA LAVAL AB 0.04% ALL NIPPON AIRWAYS CO LTD -

Member List Corporate Member List
TOP » Corporate Member List Organization / Committees Important Business Introduction Member List Annual Report Members Officers Corporate Members Association Members Overview Where We Are ABC DEF GHI JKL MNO PQR STU VWX YZ TOP Committees Corporate Member List 177 Corporate Members ABC ADEKA CORPORATION AGC Inc. Air Liquide Japan Ltd. AIR WATER INC. ARAKAWA CHEMICAL INDUSTRIES, LTD. ASAHI KASEI CORPORATION Astellas Pharma Inc. BASF Japan Ltd. Bell Polyester Products, Inc. BP Japan K.K. Canon Inc Carlit Holdings Co., Ltd. Celanese Japan Limited CENTRAL GLASS CO., LTD. Chemours Co.,Ltd. Chemours-Mitsui Fluoroproducts Co., Ltd Chevron Japan Ltd. Chugai Pharmaceutical Co., Ltd. CHUGOKU KAYAKU CO., LTD. Clariant(Japan) K.K. Connell Brothers Japan Co., Ltd. Corbion Japan K.K. Croda Japan KK DEF Dai Nippon Toryo Company, Limited DAI-ICHI KOGYO SEIYAKU CO., LTD. DAICEL CHEMICAL INDUSTRIES, LTD. Daihachi Chemical Industry Co., Ltd. DAIICHI SANKYO Co., Ltd. DAIKIN INDUSTRIES, LTD. Dainichiseika Color & Chemicals Mfg. Co., Ltd. Denka Company Limited DIC Corporation Dow Chemical Japan Limited Dow-Mitsui Polychamicals Co., Ltd. DSM K.K. Du Pont Kabushiki Kaisha Earth Chemical Co., Ltd. Eastman Chemical Japan Ltd. Eisai Co., Ltd. EMORI Infotech Co.,Ltd. Evonik Japan Co., Ltd. ExxonMobile Japan Godo Kaisha FUJIFILM Holdings Corporation FUJIFILM Wako Pure Chemical Corporation FUJIMI INCORPORATED GHI GIFU SHELLAC MANUFACTURING CO., LTD. HAKUGEN EARTH Co.,Ltd. HITACHI CHEMICAL CO., LTD. HODOGAYA CHEMICAL CO., LTD. Hokkaido Soda Co., Ltd. HOKKO CHEMICAL INDUSTRY CO., LTD. HoneyComb Techno Research Inc. Honshu Chemical Industry Co., Ltd. Idemitsu Kosan Co., Ltd. Infineum Japan Ltd. Ishihara Sangyo Kaisha, Ltd. ITOCHU Corporation JKL Japan Chemical Database Ltd.