Spotted Knapweed
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

ISTA List of Stabilized Plant Names 7Th Edition
ISTA List of Stabilized Plant Names th 7 Edition ISTA Nomenclature Committee Chair: Dr. M. Schori Published by All rights reserved. No part of this publication may be The Internation Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted Zürichstr. 50, CH-8303 Bassersdorf, Switzerland in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2020 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 ISTA List of Stabilized Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 2 7th Edition ISTA List of Stabilized Plant Names Content Preface .......................................................................................................................................................... 4 Acknowledgements ....................................................................................................................................... 6 Symbols and Abbreviations .......................................................................................................................... -

Invasive Plants in Southern Forests
Invasive Plants in Southern Forests United States Department of Agriculture A Field Guide for the Identification of Invasive PlantsSLIGHTLY inREVISED NOVEMBERSouthern 2015 Forests United States Forest Service Department Southern Research Station James H. Miller, Erwin B. Chambliss, and Nancy J. Loewenstein of Agriculture General Technical Report SRS–119 Authors: James H. Miller, Emeritus Research Ecologist, and Erwin B. Chambliss, Research Technician, Forest Available without charge from the Service, U.S. Department of Agriculture, Southern Research Station, Auburn University, AL 36849; and Southern Research Station Nancy J. Loewenstein, Research Fellow and Alabama Cooperative Extension System Specialist for Also available online at Forest Invasive Plants, School of Forestry and Wildlife Sciences, Auburn University, AL 36849. www.srs.fs.usda.gov/pubs/35292 and invasive.org, or as a free download for iPhones and iPads at the AppStore Front Cover Upper left—Chinese lespedeza (Lespedeza cuneata) infestation that developed from dormant seed in the soil seed bank after a forest thinning operation. Upper right—Kudzu (Pueraria montana) infestation within the urban-wildland interface. Lower left—Chinese privet (Ligustrum sinense) and dormant kudzu invading and replacing a pine- hardwood stand. Lower right—Cogongrass (Imperata cylindrica) infestation under mature slash pine (Pinus elliottii). Funding support for all printings provided by the Southern Research Station, Insect, Disease, and Invasive Plants Research Work Unit, and Forest Health Protection, Southern Region, Asheville, NC. First Printed April 2010 Slightly Revised February 2012 Revised August 2013 Reprinted January 2015 Slightly Revised November 2015 Southern Research Station 200 W.T. Weaver Blvd. Asheville, NC 28804 www.srs.fs.usda.gov i A Field Guide for the Identification of Invasive Plants in Southern Forests James H. -

Elymus Elymoides Crested Wheatgrass Agropyron Desertorum
Reclamation of Pipeline Right-Of-Ways on Rangelands Charlie D. Clements, James A. Young and Dan N. Harmon Introduction USDA, Agricultural Research Service 920 Valley Rd. Reno NV 89512 [email protected] In the mid 1990’s a large diameter natural gas pipeline was constructed across western Nevada. Reclamation of such right-of-ways are challenging because of both the severity and the linear nature of the disturbance. During construction, pipelines and equipment (Fig. 1) are highly visible at a landscape level to the general public, therefore attracting concerns and reclamation suggestions from a host of interest groups. The construction contractor invited us to set up plots and conduct research concerning the reclamation of their pipeline disturbance. Table 1. Plant material used in the pipeline reclamation effort. Common Name _____ Scientific Name Antelope Bitterbrush Purshia tridentata Figure 3. Lower pipeline site 6 years after the initial reclamation seeding. Basin Big Sagebrush Artemisia tridentata Basin Wildrye Leymus cinereus Big Bluegrass Poa secunda Bluebunch Wheatgrass Pseudoroegneria spicata Figure 1. Heavy equipment used in pipeline construction is very visible as well as the disturbance. Bottlebrush Squirreltail Elymus elymoides Crested Wheatgrass Agropyron desertorum Desert Needlegrass Achnatherum speciosum Idaho Fescue Festuca idahoensis ‘Immigrant’ Forage Kochia Kochia prostrata Indian Ricegrass Achnatherum hymenoides Needle-and-Threadgrass Hesperostipa comata Shadscale Atriplex confortifolia Thurber’s Needlegrass Achnatherum thurberianum Figure 4. Antelope bitterbrush, basin big sagebrush, ‘Immigrant’ forage kochia,and needle-and-thread grass were some of the planted species that successfully established. Figure 2. Pipeline site the first year after reclamation seeding effort. Results and Discussion It is a common practice to rest sites from livestock grazing for 2 years following such efforts, but Methods this is hard to do following pipeline disturbances because the disturbances are so narrow and linear in nature. -

USSR (North Caucasus, Kazakhstan Republic) July 18-August 31, 1977 U.S
PLANT GERMPLASM COLLECTION REPORT USDA-ARS FORAGE AND RANGE RESEARCH LABORATORY LOGAN, UTAH Foreign Travel to: USSR (North Caucasus, Kazakhstan Republic) July 18-August 31, 1977 U.S. Participants Douglas R. Dewey - Research Geneticist (deceased) contact Jack Staub USDA-Agricultural Research Service Logan, Utah U.S.A. A. Perry Plummer USDA-Forest Service Provo, Utah U.S.A. Laurie Law (Interpreter) USDA-ARS-IPD Washington, DC 20250 GERMPLASM ACCESSIONS Country Visited: Soviet Union -North Caucasus (Stavropol Kray) -Kazakstan Republic (Tselinograd Oblast) (Alma Ata Oblast) (Dzhambul Oblast) (Chimkent Oblast) Period of Travel: July 18 - August 31, 1977 Moscow: July 18-19 Stavropol: July 20 - August 6 Tselinograd: August 7-11 Alma Ata: August 12-16 Dzhambul: August 17-21 Chimkent: August 22-28 Moscow: August 29-31 Purpose of Trip: 1) To collect germplasm of grasses, legumes, forbs, and shrubs from natural large sites in the USSR for possible use on U.S. rangelands; 2) to establish contracts with Soviet botanists and plant breeders for the purpose of negotiating future seed exchanges. SUMMARY A 45-day plant collection expedition to the USSR by D. R. Dewey, A. P. Plummer, and Laurie Law netted about 1,100 seed collections of range-forage grasses, legumes, forbs, and shrubs. The Soviets provided land transportation and an escort of several scientists and administrators throughout the trip. The collectors were usually housed in hotels and made daily trips to collect native vegetation in surrounding areas usually within a 100-km radius. Almost 3 weeks (July 20 - August 6) were spent in the Stavropol Kray in the northern foothills of the Caucasus Mountains. -

Literature Cited Robert W. Kiger, Editor This Is a Consolidated List Of
RWKiger 26 Jul 18 Literature Cited Robert W. Kiger, Editor This is a consolidated list of all works cited in volumes 24 and 25. In citations of articles, the titles of serials are rendered in the forms recommended in G. D. R. Bridson and E. R. Smith (1991). When those forms are abbreviated, as most are, cross references to the corresponding full serial titles are interpolated here alphabetically by abbreviated form. Two or more works published in the same year by the same author or group of coauthors will be distinguished uniquely and consistently throughout all volumes of Flora of North America by lower-case letters (b, c, d, ...) suffixed to the date for the second and subsequent works in the set. The suffixes are assigned in order of editorial encounter and do not reflect chronological sequence of publication. The first work by any particular author or group from any given year carries the implicit date suffix "a"; thus, the sequence of explicit suffixes begins with "b". Works missing from any suffixed sequence here are ones cited elsewhere in the Flora that are not pertinent in these volumes. Aares, E., M. Nurminiemi, and C. Brochmann. 2000. Incongruent phylogeographies in spite of similar morphology, ecology, and distribution: Phippsia algida and P. concinna (Poaceae) in the North Atlantic region. Pl. Syst. Evol. 220: 241–261. Abh. Senckenberg. Naturf. Ges. = Abhandlungen herausgegeben von der Senckenbergischen naturforschenden Gesellschaft. Acta Biol. Cracov., Ser. Bot. = Acta Biologica Cracoviensia. Series Botanica. Acta Horti Bot. Prag. = Acta Horti Botanici Pragensis. Acta Phytotax. Geobot. = Acta Phytotaxonomica et Geobotanica. [Shokubutsu Bunrui Chiri.] Acta Phytotax. -
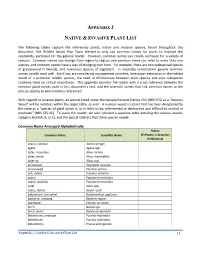
Reference Plant List
APPENDIX J NATIVE & INVASIVE PLANT LIST The following tables capture the referenced plants, native and invasive species, found throughout this document. The Wildlife Action Plan Team elected to only use common names for plants to improve the readability, particular for the general reader. However, common names can create confusion for a variety of reasons. Common names can change from region-to-region; one common name can refer to more than one species; and common names have a way of changing over time. For example, there are two widespread species of greasewood in Nevada, and numerous species of sagebrush. In everyday conversation generic common names usually work well. But if you are considering management activities, landscape restoration or the habitat needs of a particular wildlife species, the need to differentiate between plant species and even subspecies suddenly takes on critical importance. This appendix provides the reader with a cross reference between the common plant names used in this document’s text, and the scientific names that link common names to the precise species to which writers referenced. With regards to invasive plants, all species listed under the Nevada Revised Statute 555 (NRS 555) as a “Noxious Weed” will be notated, within the larger table, as such. A noxious weed is a plant that has been designated by the state as a “species of plant which is, or is likely to be, detrimental or destructive and difficult to control or eradicate” (NRS 555.05). To assist the reader, we also included a separate table detailing the noxious weeds, category level (A, B, or C), and the typical habitats that these species invade. -

ALKALI SACATON (Sporobolus Airoides) Section 7.1.4, US ARMY CORPS of ENGINEERS WILDLIFE RESOURCES MANAGEMENT MANUAL
FlSH & WlLOUFE REFERENCE LIBRARY ENVIRONMENTAL IMPACT RESEARCH PROGRAM • TECHNICAL REPORT EL-86-37 ALKALI SACATON (Sporobolus airoides) Section 7.1.4, US ARMY CORPS OF ENGINEERS WILDLIFE RESOURCES MANAGEMENT MANUAL by Clinton H. Wasser Colorado State University Fort Collins, Colorado 80523 and Phillip L. Dittberner, Donald R. Dietz US Fish and Wildlife Service Fort Collins, Colorado 80526 • July 1986 Final Report Approved For Public Release; Distribution Unlimited Prepared for DEPARTMENT OF THE ARMY US Army Corps of Engineers Washington, DC 20314-1000 Under EIRP Work Unit 31631 Monitored by Environmental Laboratory • US Army Engineer Waterways Experiment Station PO Box 631, Vicksburg, Mississippi 39180-0631 Unclassified SECURITY CLASSIFICATION OF THIS PAGE IForm Approved REPORT DOCUMENTATION PAGE OM8 No. 0704-0188 • Exp. Date: Jun 30, 1986 1a. REPORT SECURITY CLASSIFICATION 1 b. RESTRICTIVE MARKINGS Uncla<:<:ified 2a. SECURITY CLASSIFICATION AUTHORITY 3. DISTRIBUTION I AVAILABILITY OF REPORT Approved for public release; 2b. DECLASSIFICATION I DOWNGRADING SCHEDULE distribution unlimited. 4. PERFORMING ORGANIZATION REPORT NUMBER(S) 5. MONITORING ORGANIZATION REPORT NUMBER(S) Technical Report EL-86-37 6a. NAME OF PERFORMING ORGANIZATION 6b. OFFICE SYMBOL la. NAME OF MONITORING ORGANIZATION See reverse (If applicable) USAEWES Environmental Laboratorv 6c. ADDRESS (City, State, and ZIP Code) 7b. ADDRESS (City, State, and ZIP Code) See reverse PO Box 631 Vicksburg, MS 39180-0631 Ba. NAME OF FUNDING /SPONSORING Sb. OFFICE SYMBOL 9. PROCUREMENT INSTRUMENT IDENTIFICATION NUMBER ORGANIZATION (If applicable) US Army Corps of Engineers Sc. ADDRESS (City, State, and ZIP Code) 10. SOURCE OF FUNDING NUMBERS PROGRAM PROJECT TASK WORK UNIT ELEMENT NO. NO. NO. ACCESSION NO Washington, DC 20314-1000 EIRP 316'H 11. -

Discovery, Expression Profiling, and Evolutionary Analysis Of
DISCOVERY, EXPRESSION PROFILING, AND EVOLUTIONARY ANALYSIS OF CYNODON EXPRESSED SEQUENCE TAGS by CHANGSOO KIM (Under the Direction of Andrew H. Paterson) ABSTRACT Bermudagrass (Cynodon dactylon) is a major turfgrass species for sports fields, lawns, parks, golf courses, and general utility turfs in tropical and subtropical regions. Despite its ecological importance, much of its study has been dependent upon classical approaches. Information about Bermudagrass at the molecular level has been deficient although molecular information for other plants has been accumulated for the last two dacades. In the current study, we constructed a normalized cDNA library from leaf tissue of Bermudagrass in order to expand our knowledge of its transcriptome. We sequenced and annotated 15,588 expressed sequence tags (ESTs), which were deposited in the National Center for Biotechnology Information (NCBI) to be shared with other scientists. We also conducted cDNA array hybridization (macroarray) to profile genes responding to drought stress. A total of 120 and 69 genes were identified as up- and down-regulated, respectively. BLASTX annotation suggested that up-regulated genes may be involved in osmotic adjustment, signal transduction pathways, protein repair systems, and removal of toxins, while down-regulated genes were mostly related to basic plant metabolism such as photosynthesis and glycolysis. Using the cDNA sequences, we performed a comparative genomic study to gain new insight into the evolution of Bermudagrass. Results suggested that the common ancestor of the grass family experienced a whole genome duplication event at ca. 50.0 ~ 65.4 million years ago (MYA), before the divergence of the PACC and BEP clades at ca. 42.3 ~ 50.0 MYA. -

Crested Wheatgrass Agropyron Desertorum (A.Cristatum)
Crested Wheatgrass Agropyron desertorum (A.cristatum) Crested Wheatgrass is a cool season, vigorous growing, and drought tolerant, winter hardy, perennial bunchgrass which grows to a height of 1 to 3 feet. It’s dark green leaf blades taper to a sharp point and extend sharply upward from the base during the early part of the growing season. The leaf sheaths, which wrap tightly around the stems, are longer than the internodes, and have a smooth surface. The seed heads are approximately two inches long; have an unusual flat cockscomb or crested shape; and taper toward the tip, with the axis showing fine hairs. The grass has an extensive fibrous root system which penetrates as much as six feet. This gives the plant an advantage over weeds and enables it to do well in areas of as little as 12 inches of annual rainfall. Its drought tolerance, winter hardiness, and ability to withstand grazing, combined with high palatability and nutritious forage make it a valuable plant. There are two distinct species of crested wheatgrass – Standard (A. desertorum), and Fairway – A. cristatum). Crested wheatgrass should generally be seeded below 7,000 feet elevation. Crested wheatgrass does well on shallow to deep, moderately course to fine textured, moderately well to well drained soils. Under saline conditions, vigor and production are reduced. Seeding Rate: 5 to 7 PLS Lbs/ Ac Height Range (inches): 12 – 36 Seeds Per Pound: 311,200 Taxonomy Species: Agropyron cristatum or Agropyron desertorum Genus: Agropyron Gaertn. Family: Poaceae Order: Cyperales Subclass: Commelinidae Class: Liliopsida Division: Magnoliophyta Subkingdom: Tracheobionta Kingdom: Plantae Peoria, AZ 85382 Phone: 623-572-4907 Fax: 623-566-1436 www.arizonaseed.com . -

GRASSES AS INVASIVE SPECIES Clay Antieau, MS, Phc Botanist, Horticulturist, Environmental Educator
GRASSES AS INVASIVE SPECIES Clay Antieau, MS, PhC Botanist, Horticulturist, Environmental Educator WFCA Eighth Western Native Plant Conference November 2019 THE GRASS FAMILY (Poaceae) • Genera : 700 – 800 • Species: 7,500 – 11,000 (4th largest) • Comparable in size to Aves (Birds); ~ twice as large as Mammalia; half the size of Orchid or Aster families • First appeared in pollen record 55 – 70 mya (Paleocene) • Only angiosperm family found natively on all seven continents (Deschampsia Antarctica) • Includes cereal crops (forage, food, sugar, beer...); many ecological dominants • All major civilizations developed around cultivated grasses (Asia to Middle East to New World) Among the World’s Worst Invasives: Grasses • Imperata cylindrica (cogon grass) • Phragmites australis (common reed) • Phalaris arundinacea (reed canarygrass) • Sorghum halepense (Johnson grass) • Spartina species and hybrids (cordgrasses) • Cortaderia species and hybrids (pampas grass) • Arundo donax (giant reed) • Microstegium vimineum (Japanese stilt grass) • Bromus tectorum (cheat) • Taeniatherum caput-medusae (medusahead) • Cynodon dactylon (Bermuda grass) • Neyraudia reynaudiana (silk reed) Important North American Invasive Grasses (adapted from www.fs.fed.us/database/feis) Aegilops cylindricus (goat grass) Eragrostis curvula (weeping lovegrass) Aegilops triuncialis (barbed goat grass) Eragrostis lehmanniana (Lehmann love Agropyron desertorum (desert wheat grass) grass) Elytrigia repens (quack grass) Agropyron cristatum (crested wheat Festuca arundinacea (tall fescue) -

Ecology of the Black-Tailed Jack Rabbit Near a Solid Radioactive Waste Disposal Site in Southeastern Idaho
University of Montana ScholarWorks at University of Montana Graduate Student Theses, Dissertations, & Professional Papers Graduate School 1987 Ecology of the black-tailed jack rabbit near a solid radioactive waste disposal site in southeastern Idaho John C. Grant The University of Montana Follow this and additional works at: https://scholarworks.umt.edu/etd Let us know how access to this document benefits ou.y Recommended Citation Grant, John C., "Ecology of the black-tailed jack rabbit near a solid radioactive waste disposal site in southeastern Idaho" (1987). Graduate Student Theses, Dissertations, & Professional Papers. 7052. https://scholarworks.umt.edu/etd/7052 This Thesis is brought to you for free and open access by the Graduate School at ScholarWorks at University of Montana. It has been accepted for inclusion in Graduate Student Theses, Dissertations, & Professional Papers by an authorized administrator of ScholarWorks at University of Montana. For more information, please contact [email protected]. COPYRIGHT ACT OF 1976 T h i s i s an unpublished m a n u s c r ip t in w h ic h c o p y r ig h t SUBSISTS. An y f u r t h e r r e p r in t in g o f i t s c o n t e n t s m u s t b e APPROVED BY THE AUTHOR. Ma n s f ie l d L ib r a r y Un iv e r s i t y o f Mo n t a n a Da t e : ______ I 9 S '?__ Reproduced with permission of the copyright owner. -

C3 Grasses Have Higher Nutritional Quality Than C4 Grasses Under Ambient and Elevated Atmospheric CO2
Global Change Biology (2004) 10, 1565–1575, doi: 10.1111/j.1365-2486.2004.00833.x C3 grasses have higher nutritional quality than C4 grasses under ambient and elevated atmospheric CO2 RAYMOND V. BARBEHENN*,ZHONGCHENw ,DAVIDN.KAROWEz and A N G E L A SPICKARD§ *Departments of Molecular, Cellular and Developmental Biology and Ecology and Evolutionary Biology, University of Michigan, Ann Arbor, MI 48109-1048, USA, wSchool of Forestry, Northern Arizona University, Flagstaff, AZ 86011-5018, USA, zWestern Michigan University, Department of Biological Sciences, Kalamazoo, MI 49008-5410, USA, §Environmental Protection Agency, Ann Arbor, MI 48105, USA Abstract Grasses with the C3 photosynthetic pathway are commonly considered to be more nutritious host plants than C4 grasses, but the nutritional quality of C3 grasses is also more greatly impacted by elevated atmospheric CO2 than is that of C4 grasses; C3 grasses produce greater amounts of nonstructural carbohydrates and have greater declines in their nitrogen content than do C4 grasses under elevated CO2. Will C3 grasses remain nutritionally superior to C4 grasses under elevated CO2 levels? We addressed this question by determining whether levels of protein in C3 grasses decline to similar levels as in C4 grasses, and whether total carbohydrate : protein ratios become similar in C3 and C4 grasses under elevated CO2. In addition, we tested the hypothesis that, among the nonstructural carbohydrates in C3 grasses, levels of fructan respond most strongly to elevated CO2. Five C3 and five C4 grass species were grown from seed in outdoor open- top chambers at ambient (370 ppm) or elevated (740 ppm) CO2 for 2 months.