Meningeal Defects Alter the Tangential Migration Of
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection
Distance Learning Program Anatomy of the Human Brain/Sheep Brain Dissection This guide is for middle and high school students participating in AIMS Anatomy of the Human Brain and Sheep Brain Dissections. Programs will be presented by an AIMS Anatomy Specialist. In this activity students will become more familiar with the anatomical structures of the human brain by observing, studying, and examining human specimens. The primary focus is on the anatomy, function, and pathology. Those students participating in Sheep Brain Dissections will have the opportunity to dissect and compare anatomical structures. At the end of this document, you will find anatomical diagrams, vocabulary review, and pre/post tests for your students. The following topics will be covered: 1. The neurons and supporting cells of the nervous system 2. Organization of the nervous system (the central and peripheral nervous systems) 4. Protective coverings of the brain 5. Brain Anatomy, including cerebral hemispheres, cerebellum and brain stem 6. Spinal Cord Anatomy 7. Cranial and spinal nerves Objectives: The student will be able to: 1. Define the selected terms associated with the human brain and spinal cord; 2. Identify the protective structures of the brain; 3. Identify the four lobes of the brain; 4. Explain the correlation between brain surface area, structure and brain function. 5. Discuss common neurological disorders and treatments. 6. Describe the effects of drug and alcohol on the brain. 7. Correctly label a diagram of the human brain National Science Education -

A Cellular Atlas of the Developing Meninges Reveals Meningeal Fibroblast Diversity and Function
bioRxiv preprint doi: https://doi.org/10.1101/648642; this version posted May 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 2 3 4 Title: A cellular atlas of the developing meninges reveals meningeal fibroblast diversity and function 5 6 Authors: John DeSisto1,2,3,, Rebecca O’Rourke2, Stephanie Bonney1,3, Hannah E. Jones1,3, Fabien 7 Guimiot4, Kenneth L. Jones2 and Julie A. Siegenthaler1,3,5 8 9 1Department of Pediatrics Section of Developmental Biology, 2Department of Pediatrics Section of 10 Section of Hematology, Oncology, Bone Marrow Transplant, 3Cell Biology, Stem Cells and Development 11 Graduate Program, University of Colorado, Anschutz Medical Campus, Aurora, CO 80045 USA, 12 4INSERM UMR 1141, Hôpital Robert Debré, 75019 Paris, France. 13 14 5Corresponding Author: 15 Julie A. Siegenthaler, PhD 16 University of Colorado, School of Medicine 17 Department of Pediatrics 18 12800 East 19th Ave MS-8313 19 Aurora, CO 80045 USA 20 Telephone #: 303-724-3123 21 E-mail: [email protected] 22 23 Key words (3-6 words): brain development, meninges, pial basement membrane, retinoic acid, human 24 meninges bioRxiv preprint doi: https://doi.org/10.1101/648642; this version posted May 24, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 25 Abstract 26 The meninges, a multilayered structure that encases the CNS, is composed mostly of fibroblasts, 27 along with vascular and immune cells. -

Lecture 4: the Meninges And
1/1/2016 Introduction • Protection of the brain – Bone (skull) The Nervous System – Membranes (meninges) – Watery cushion (cerebrospinal fluid) – Blood-brain barrier (astrocytes) Meninges CSF The Meninges The Meninges • Series of membranes • Three layers • Cover and protect the CNS – Dura mater • Anchor and cushion the brain – Arachnoid mater – • Contain cerebrospinal fluid (CSF) Pia mater The Meninges • Dura mater – “Tough mother” Skin of scalp Periosteum – Strongest meninx Bone of skull Periosteal Dura – Fibrous connective tissue Meningeal mater Superior Arachnoid mater – sagittal sinus Pia mater Limit excessive movement of the brain Subdural Arachnoid villus – space Blood vessel Forms partitions in the skull Subarachnoid Falx cerebri space (in longitudinal fissure only) Figure 12.24 1 1/1/2016 Superior The Meninges sagittal sinus Falx cerebri • Arachnoid mater – “Spider mother” Straight sinus – Middle layer with weblike extensions Crista galli – Separated from the dura mater by the subdural space of the Tentorium ethmoid cerebelli – Subarachnoid space contains CSF and blood vessels bone Falx Pituitary cerebelli gland (a) Dural septa Figure 12.25a The Meninges • Pia mater – “Gentle mother” – Connected to the dura mater by projections from the arachnoid mater – Layer of delicate vascularized connective tissue – Clings tightly to the brain T Meningitis TT121212 Ligamentum flavumflavumflavum L • LL555 Lumbar puncture Inflammation of meninges needle entering subarachnoid • May be bacterial or viral spacespacespace LLL444 • Diagnosed by -

Carcinomatosis of the Meninges of the Spinal Cord and Base of the Brain, Without Involvement of the Parenchyma, Secondary to Carcinoma of the Lung
CARCINOMATOSIS OF THE MENINGES OF THE SPINAL CORD AND BASE OF THE BRAIN, WITHOUT INVOLVEMENT OF THE PARENCHYMA, SECONDARY TO CARCINOMA OF THE LUNG BERNARD J. ALPERS, M.D., A~D O. NORRIS SMITH, M.D. (From the Laboratories of Neurosurgery and Pathology in the Hospital of the University of Pennsylvania) Metastatic carcinoma of the nervous system limited to the meninges IS rare, but it has been frequently described. Carcinomatosis of this sort is usually confined to the meninges over the cerebral hemispheres and only occa sionally spreads to the spinal membranes. Meningeal carcinomatosis con fined to the spinal cord and to a portion of the base of the brain without in volvement of the cerebrum itself is so unusual that the following case is pre sented as an addition to our knowledge concerning metastatic carcinoma of the central nervous system. REPORT OF CASE CASE No. 36-23924: Weakness five months; lumbar pain one month; mass in left upper lobe demonstrable roentgenographically; severe pain in legs; wasting and progressive weak ness suggestive of spinal cord lesion; flaccid paralysis; fibrillary tremors in trunk and limb muscles; diminished lower abdominal and leg reflexes; inconclusive Queckenstedt test; pleocytosis in spinal fluid. Necropsy findings: mass in left lung, probably primary carci noma; meningeal carcinomatosis oj spinal cord membranes and membranes at base of brain; no tumor in cord or brain. History: J. R., a fifty-six-year-old white farmer, recently employed as a church sexton, was admitted to the medical service of Dr. Alfred Stengel on Sept. 12, 1936, complaining of lower back pain which had confined him to bed for a month. -

Review of Spinal Cord Basics of Neuroanatomy Brain Meninges
Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Parts of Nervous System Review of Spinal Cord with Basics of Neuroanatomy Brain Meninges Prof. D.H. Pauža Neurons and Neuroglia Neuron Human brain contains per 1011-12 (trillions) neurons Body (soma) Perikaryon Nissl substance or Tigroid Dendrites Axon Myelin Terminals Synapses Neuronal types Unipolar, pseudounipolar, bipolar, multipolar Afferent (sensory, centripetal) Efferent (motor, centrifugal, effector) Associate (interneurons) Synapse Presynaptic membrane Postsynaptic membrane, receptors Synaptic cleft Synaptic vesicles, neuromediator Mitochondria In human brain – neurons 1011 (100 trillions) Synapses – 1015 (quadrillions) Neuromediators •Acetylcholine •Noradrenaline •Serotonin •GABA •Endorphin •Encephalin •P substance •Neuronal nitric oxide Adrenergic nerve ending. There are many 50-nm-diameter vesicles (arrow) with dark, electron-dense cores containing norepinephrine. x40,000. Cell Types of Neuroglia Astrocytes - Oligodendrocytes – Ependimocytes - Microglia Astrocytes – a part of hemoencephalic barrier Oligodendrocytes Ependimocytes and microglial cells Microglia represent the endogenous brain defense and immune system, which is responsible for CNS protection against various types of pathogenic factors. After invading the CNS, microglial precursors disseminate relatively homogeneously throughout the neural tissue and acquire a specific phenotype, which clearly distinguish them from their precursors, the blood-derived monocytes. The ´resting´ microglia -

Pal Worksheet: Nervous System, Brain, Brainstem, Cerebellum, Wk9 Brain 1
Pal worksheet: nervous system, brain, brainstem, cerebellum, wk9 Brain 1. Describe the relationship of white matter to grey matter in the brain – how does this differ from spinal cord? 2. What does white matter consist of, and what is its function - are there any differences between CNS and PNS in terms of this white matter? 3. What does grey matter consist of? Grey matter for the most part consist of the cell bodies of neurons, as well as interneurons and supporting cells – 4. Draw a diagram of the brain from the side: label important sulci and gyri – label the major lobes of the brain – on each lobe list at least two functions that are known to require that particular lobe – label primary motor cortex, primary somatosensory cortex, lateral fissure and central sulcus, primary visual cortex, primary auditory cortex 5. Create an illustration of the meninges of the brain, include any important spaces and traversing structures – make sure the drawing makes clear how sinuses are formed 6. Draw a picture of the ventricles of the brain and include important passageways 7. What flows through the ventricular system? Where does it travel after it exits the ventricular system? Where does it go to once it finishes circulating around the brain and spinal cord? Brainstem 8. List 5 major involuntary/autonomic functions that are regulated/controlled in whole or in part by the brainstem 9. Name the three regions of the brainstem and describe a function that is associated with each region: a. b. c. Cerebellum 10. The cerebellum is involved in motor control. -

Spinal Meninges Neuroscience Fundamentals > Regional Neuroscience > Regional Neuroscience
Spinal Meninges Neuroscience Fundamentals > Regional Neuroscience > Regional Neuroscience SPINAL MENINGES GENERAL ANATOMY Meningeal Layers From outside to inside • Dura mater • Arachnoid mater • Pia mater Meningeal spaces From outside to inside • Epidural (above the dura) - See: epidural hematoma and spinal cord compression from epidural abscess • Subdural (below the dura) - See: subdural hematoma • Subarachnoid (below the arachnoid mater) - See: subarachnoid hemorrhage Spinal canal Key Anatomy • Vertebral body (anteriorly) • Vertebral arch (posteriorly). • Vertebral foramen within the vertebral arch. MENINGEAL LAYERS 1 / 4 • Dura mater forms a thick ring within the spinal canal. • The dural root sheath (aka dural root sleeve) is the dural investment that follows nerve roots into the intervertebral foramen. • The arachnoid mater runs underneath the dura (we lose sight of it under the dural root sheath). • The pia mater directly adheres to the spinal cord and nerve roots, and so it takes the shape of those structures. MENINGEAL SPACES • The epidural space forms external to the dura mater, internal to the vertebral foramen. • The subdural space lies between the dura and arachnoid mater layers. • The subarachnoid space lies between the arachnoid and pia mater layers. CRANIAL VS SPINAL MENINGES  Cranial Meninges • Epidural is a potential space, so it's not a typical disease site unless in the setting of high pressure middle meningeal artery rupture or from traumatic defect. • Subdural is a potential space but bridging veins (those that pass from the subarachnoid space into the dural venous sinuses) can tear, so it is a common site of hematoma. • Subarachnoid space is an actual space and is a site of hemorrhage and infection, for example. -

MENINGES and CEREBROSPINAL FLUID' by LEWIS H
MENINGES AND CEREBROSPINAL FLUID' By LEWIS H. WEED Department of Anatomy, John Hopkins University THE divorce of structure from function is particularly difficult in any ana- tomical study: it was only 85 years ago that the two subjects of morphology and physiology were considered to justify separate departments as academic disciplines. But with this cleavage which fortunately has not at any time been a rigid one, only certain investigations could go forward without loss of in- spiration and interpretation when studied apart from the sister science; other researches were enormously hampered and could be attacked only with due regard to structure and function. So it is without apologies that I begin the presentation of the problem of the coverings of the central nervous system -coverings which encompass a characteristic body fluid. Here then is a problem of membranes serving to contain a clear, limpid liquid as a sac might hold it. Immediately many questions of biological significance are at hand: how does it happen that these structures retain fluid; where does the fluid come from; where does it go; is the fluid constantly produced or is it an inert, non-circu- lating medium; is the fluid under pressure above that of the atmosphere; does it move about with changes in the animal body?-but the list of problems springing into one's mind grows too long. Knowledge regarding these many questions has progressed since the first accounts of hydrocephalus were given by writers in the Hippocratic corpus, since discovery of the normal ventricular fluid in Galen's time, since its meningeal existence was first uncovered by Valsalva (1911) and advanced by Cotugno (1779), since the first adequate description by Magendie (1825) 100 years ago. -
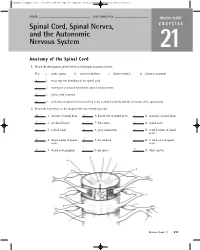
Spinal Cord, Spinal Nerves, and the Autonomic Nervous System
ighapmLre21pg211_216 5/12/04 2:24 PM Page 211 impos03 302:bjighapmL:ighapmLrevshts:layouts: NAME ___________________________________ LAB TIME/DATE _______________________ REVIEW SHEET Spinal Cord, Spinal Nerves, exercise and the Autonomic Nervous System 21 Anatomy of the Spinal Cord 1. Match the descriptions given below to the proper anatomical term: Key: a. cauda equina b. conus medullaris c. filum terminale d. foramen magnum d 1. most superior boundary of the spinal cord c 2. meningeal extension beyond the spinal cord terminus b 3. spinal cord terminus a 4. collection of spinal nerves traveling in the vertebral canal below the terminus of the spinal cord 2. Match the key letters on the diagram with the following terms. m 1. anterior (ventral) hornn 6. dorsal root of spinal nervec 11. posterior (dorsal) horn k 2. arachnoid materj 7. dura materf 12. spinal nerve a 3. central canalo 8. gray commissure i 13. ventral ramus of spinal nerve h 4. dorsal ramus of spinald 9. lateral horne 14. ventral root of spinal nerve nerve g l 5. dorsal root ganglion 10. pia materb 15. white matter o a b n c m d e l f g k h j i Review Sheet 21 211 ighapmLre21pg211_216 5/12/04 2:24 PM Page 212 impos03 302:bjighapmL:ighapmLrevshts:layouts: 3. Choose the proper answer from the following key to respond to the descriptions relating to spinal cord anatomy. Key: a. afferent b. efferent c. both afferent and efferent d. association d 1. neuron type found in posterior hornb 4. fiber type in ventral root b 2. -

The Meninges As Barriers and Facilitators for the Movement of Fluid, Cells and Pathogens Related to the Rodent and Human CNS
The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS Weller, Roy O.; Sharp, Matthew M.; Christodoulides, Myron; Carare, Roxana O.; Møllgård, Kjeld Published in: Acta Neuropathologica DOI: 10.1007/s00401-018-1809-z Publication date: 2018 Document version Publisher's PDF, also known as Version of record Document license: CC BY Citation for published version (APA): Weller, R. O., Sharp, M. M., Christodoulides, M., Carare, R. O., & Møllgård, K. (2018). The meninges as barriers and facilitators for the movement of fluid, cells and pathogens related to the rodent and human CNS. Acta Neuropathologica, 135(3), 363-385. https://doi.org/10.1007/s00401-018-1809-z Download date: 28. Sep. 2021 Acta Neuropathologica (2018) 135:363–385 https://doi.org/10.1007/s00401-018-1809-z REVIEW The meninges as barriers and facilitators for the movement of fuid, cells and pathogens related to the rodent and human CNS Roy O. Weller1 · Matthew M. Sharp1 · Myron Christodoulides2 · Roxana O. Carare1 · Kjeld Møllgård3 Received: 5 November 2017 / Revised: 2 January 2018 / Accepted: 15 January 2018 / Published online: 24 January 2018 © The Author(s) 2018. This article is an open access publication Abstract Meninges that surround the CNS consist of an outer fbrous sheet of dura mater (pachymeninx) that is also the inner peri- osteum of the skull. Underlying the dura are the arachnoid and pia mater (leptomeninges) that form the boundaries of the subarachnoid space. In this review we (1) examine the development of leptomeninges and their role as barriers and facilita- tors in the foetal CNS. -

The Anatomy of the Murine Cortical Meninges Revisited for Intravital Imaging, Immunology, and Clearance of Waste from the Brain
This is a repository copy of Where are we? : The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/133774/ Version: Accepted Version Article: Coles, Jonathan A, Myburgh, Elmarie orcid.org/0000-0002-2007-5902, Brewer, James M et al. (1 more author) (2017) Where are we? : The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Progress in neurobiology. pp. 107-148. ISSN 1873-5118 https://doi.org/10.1016/j.pneurobio.2017.05.002 Reuse This article is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs (CC BY-NC-ND) licence. This licence only allows you to download this work and share it with others as long as you credit the authors, but you can’t change the article in any way or use it commercially. More information and the full terms of the licence here: https://creativecommons.org/licenses/ Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Progress in Neurobiology Accepted for publication 8 May 2017. Review article Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Jonathan A. Colesa,*, Elmarie Myburghb, James M. -

Fig. 13.1 Copyright © Mcgraw-Hill Education
Fig. 13.1 Copyright © McGraw-Hill Education. Permission required for reproduction or display. C1 Cervical Cervical enlargement spinal nerves C7 Dural sheath Subarachnoid space Thoracic spinal Spinal cord nerves Vertebra (cut) Lumbar Spinal nerve enlargement T12 Spinal nerve rootlets Medullary cone Posterior median sulcus Lumbar Subarachnoid space Cauda equina spinal nerves Epidural space Posterior root ganglion L5 Rib Arachnoid mater Terminal Sacral Dura mater filum spinal nerves S5 Col (b) (a) 1 Fig. 13.2 Copyright © McGraw-Hill Education. Permission required for reproduction or display. Posterior Spinous process of vertebra Meninges: Dura mater (dural sheath) Arachnoid mater Fat in epidural space Pia mater Subarachnoid space Spinal cord Denticulate ligament Posterior root ganglion Spinal nerve Vertebral body (a) Spinal cord and vertebra (cervical) Anterior Posterior Gray matter: Central canal median sulcus White matter: Posterior horn Posterior column Gray commissure Lateral column Lateral horn Anterior column Anterior horn Posterior root of spinal nerve Posterior root ganglion Spinal nerve Anterior median fissure Anterior root of spinal nerve Meninges: Pia mater Arachnoid mater Dura mater (dural sheath) (b) Spinal cord and meninges (thoracic) (c) Lumbar spinal cord c: ©Ed Reschke/Getty Images 2 Table 13.1 3 Fig. 13.4 Copyright © McGraw-Hill Education. Permission required for reproduction or display. Ascending Descending tracts tracts Posterior column: Gracile fasciculus Cuneate fasciculus Anterior corticospinal tract Lateral Posterior spinocerebellar tract corticospinal tract Lateral reticulospinal tract Anterior spinocerebellar tract Tectospinal tract Anterolateral system (containing Medial reticulospinal tract spinothalamic and spinoreticular tracts) Lateral vestibulospinal tract Medial vestibulospinal tract 4 Fig. 13.5 Copyright © McGraw-Hill Education. Permission required for reproduction or display.