Organic Molecules in the Sheepbed Mudstone, Gale Crater, Mars
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Chemical Variations in Yellowknife Bay Formation Sedimentary Rocks
PUBLICATIONS Journal of Geophysical Research: Planets RESEARCH ARTICLE Chemical variations in Yellowknife Bay formation 10.1002/2014JE004681 sedimentary rocks analyzed by ChemCam Special Section: on board the Curiosity rover on Mars Results from the first 360 Sols of the Mars Science Laboratory N. Mangold1, O. Forni2, G. Dromart3, K. Stack4, R. C. Wiens5, O. Gasnault2, D. Y. Sumner6, M. Nachon1, Mission: Bradbury Landing P.-Y. Meslin2, R. B. Anderson7, B. Barraclough4, J. F. Bell III8, G. Berger2, D. L. Blaney9, J. C. Bridges10, through Yellowknife Bay F. Calef9, B. Clark11, S. M. Clegg5, A. Cousin5, L. Edgar8, K. Edgett12, B. Ehlmann4, C. Fabre13, M. Fisk14, J. Grotzinger4, S. Gupta15, K. E. Herkenhoff7, J. Hurowitz16, J. R. Johnson17, L. C. Kah18, N. Lanza19, Key Points: 2 1 20 21 12 16 2 • J. Lasue , S. Le Mouélic , R. Léveillé , E. Lewin , M. Malin , S. McLennan , S. Maurice , Fluvial sandstones analyzed by 22 22 23 19 19 24 25 ChemCam display subtle chemical N. Melikechi , A. Mezzacappa , R. Milliken , H. Newsom , A. Ollila , S. K. Rowland , V. Sautter , variations M. Schmidt26, S. Schröder2,C.d’Uston2, D. Vaniman27, and R. Williams27 • Combined analysis of chemistry and texture highlights the role of 1Laboratoire de Planétologie et Géodynamique de Nantes, CNRS, Université de Nantes, Nantes, France, 2Institut de Recherche diagenesis en Astrophysique et Planétologie, CNRS/Université de Toulouse, UPS-OMP, Toulouse, France, 3Laboratoire de Géologie de • Distinct chemistry in upper layers 4 5 suggests distinct setting and/or Lyon, Université de Lyon, Lyon, France, California Institute of Technology, Pasadena, California, USA, Los Alamos National 6 source Laboratory, Los Alamos, New Mexico, USA, Earth and Planetary Sciences, University of California, Davis, California, USA, 7Astrogeology Science Center, U.S. -

Orbital Evidence for More Widespread Carbonate- 10.1002/2015JE004972 Bearing Rocks on Mars Key Point: James J
PUBLICATIONS Journal of Geophysical Research: Planets RESEARCH ARTICLE Orbital evidence for more widespread carbonate- 10.1002/2015JE004972 bearing rocks on Mars Key Point: James J. Wray1, Scott L. Murchie2, Janice L. Bishop3, Bethany L. Ehlmann4, Ralph E. Milliken5, • Carbonates coexist with phyllosili- 1 2 6 cates in exhumed Noachian rocks in Mary Beth Wilhelm , Kimberly D. Seelos , and Matthew Chojnacki several regions of Mars 1School of Earth and Atmospheric Sciences, Georgia Institute of Technology, Atlanta, Georgia, USA, 2The Johns Hopkins University/Applied Physics Laboratory, Laurel, Maryland, USA, 3SETI Institute, Mountain View, California, USA, 4Division of Geological and Planetary Sciences, California Institute of Technology, Pasadena, California, USA, 5Department of Geological Sciences, Brown Correspondence to: University, Providence, Rhode Island, USA, 6Lunar and Planetary Laboratory, University of Arizona, Tucson, Arizona, USA J. J. Wray, [email protected] Abstract Carbonates are key minerals for understanding ancient Martian environments because they Citation: are indicators of potentially habitable, neutral-to-alkaline water and may be an important reservoir for Wray, J. J., S. L. Murchie, J. L. Bishop, paleoatmospheric CO2. Previous remote sensing studies have identified mostly Mg-rich carbonates, both in B. L. Ehlmann, R. E. Milliken, M. B. Wilhelm, Martian dust and in a Late Noachian rock unit circumferential to the Isidis basin. Here we report evidence for older K. D. Seelos, and M. Chojnacki (2016), Orbital evidence for more widespread Fe- and/or Ca-rich carbonates exposed from the subsurface by impact craters and troughs. These carbonates carbonate-bearing rocks on Mars, are found in and around the Huygens basin northwest of Hellas, in western Noachis Terra between the Argyre – J. -

Mineralogy and Geochemistry of Carbonaceous Mudstone As a Vector to Ore: a Case Study at the Lagunas Norte High-Sulfidation Gold Deposit, Peru
MINERALOGY AND GEOCHEMISTRY OF CARBONACEOUS MUDSTONE AS A VECTOR TO ORE: A CASE STUDY AT THE LAGUNAS NORTE HIGH-SULFIDATION GOLD DEPOSIT, PERU by Harry Hanneman A thesis submitted to the Faculty and Board of Trustees of the Colorado School of Mines in partial fulfillment of the requirements for the degree of Master of Science (Geology). Golden, Colorado Date ________________ Signed: ________________________ Harry Hanneman Signed: ________________________ Dr. Thomas Monecke Thesis Advisor Golden, Colorado Date ________________ Signed: ________________________ Dr. Paul Santi Professor and Head Department of Geology and Geological Engineering ii ABSTRACT The Lagunas Norte Au deposit in the Alto Chicama district of Peru is a ~14 Moz high- sulfidation epithermal deposit that is hosted by an atypical host-rock succession for this deposit type. Approximately 80% of the ore body is contained in the Lower Cretaceous Chimu Formation, which is composed of quartz arenite with interbedded carbonaceous mudstone, siltstone, and coal seams. The remainder of the ore is hosted by the Miocene volcanic rocks of the Calipuy Group, forming an irregular and thin veneer on the deformed sedimentary rocks of the basement. The host rock succession of the Lagunas Norte deposit has been affected by widespread hydrothermal alteration. The alteration is cryptic within most of sedimentary rocks as the quartz arenite was largely inert to alteration by the strongly acidic fluids. Vuggy textures associated with residual quartz alteration can only be recognized in the overlying Miocene volcanic rocks. However, the present study shows that mudstone of the Chimu Formation records acid-type alteration due to its originally high clay mineral content. -
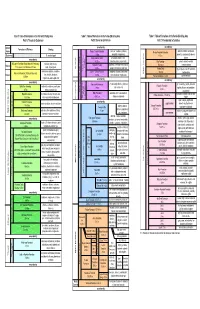
Table of Formations
Table 1: Table of Formations in the Fernie-Elk Valley Area Table 1: Table of Formations in the Fernie-Elk Valley Area Table 1: Table of Formations in the Fernie-Elk Valley Area Part A: Triassic to Quaternary Part B: Devonian to Permian Part C: Precambrian to Cambrian System/ unconformity unconformity Formation and Thickness Lithology Series Ranger Canyon Formation chert, with sandstone, siltstone, Windsor Mountain Formation dolomite-mottled limestone and phosphatic conglomerate Quaternary till, sand and gravel 0-36m 0-70 m dolomite; calcareous silty dolomite phosphatic siltstone, chert, at base unconformity Ross Creek Formation 0-150 m limestone, basal conglomerate Elko Formation dolomite, dolomite-mottled St.Eugene Formation (Rocky Mountain Trench only) colluvium, fanglomerate, Miocene Telford Formation fossilliferous limestone and dolomite, 150-160 m limestone at base 50 m exposed, total thickness unknown sand, silt and gravel 0-500m Permian 0-240 m sandy and silty in part, and chert Gordon Shale greyish green shale,with sandstone varicoloured sandstone, mudstone, Ishbel Group Johnson Canyon Formation phosphatic siltstones, chert, basal 45-90 m and limestone Kishenehn Formation (Flathead Valley only) clay, oil shale, limestone, Middle Cambrian Oligocene 0-60 m chert-phosphate conglomerate Flathead Sandstone 2-45 m quartz sandstone 0-5000m conglomerate and megabreccia unconformity unconformity unconformity silty and sandy dolomite, siltstones green and grey, argillite, dolomitic Kananaskis Formation Roosville Formation Belly River Formation -

Encounters with an Unearthly Mudstone
ENCOUNTERS WITH AN UNEARTHLY MUDSTONE Understanding the First Mudstone Found on Mars By Juergen Schieber1, David Bish1, Max Coleman2, Mark Reed3, Elisabeth M. Hausrath4, John Cosgrove5, Sanjeev Gupta5, Michelle E. Minitti6, Ken Edgett7, and Mike Malin7 1 = Indiana University, Bloomington, IN; 2 = JPL, Pasadena, CA; 3 = University of Oregon, OR; 4 = University of Nevada Las Vegas; NV; 5 = Imperial College, London, UK; 6 = Planetary Science Institute, Tucson, AZ; 7 = Malin Space Science Systems, San Diego, CA Paper Structure Abstract Introduction Doing Geology on Mars – Limitations of the Rover Geologist Large Scale Stratigraphic Relationships Methods Eolian Abrasion of Mudstones as a Source of Insight Physical Sedimentology of the Sheepbed Mudstone Subdivisions: Layer Characteristics: Interpretation of Sheepbed mudstone stratification: Polygonal Cracks and their Significance Perspectives on the Origin of Clays APXS Analyses: XRD Analyses: Modeling Diagenetic Clay Formation: Kinetics of Clay Formation: Diagenesis Early Diagenesis: Nodules and Raised Ridges: Diagenetic Beds: Additional Observations and Thoughts on Nodules and Raised Ridges: The Cement Mystery: A Model for Early Diagenesis: Late Diagenesis: Straight Veins: Knobbly Veins: Horizontal Veins at Lithologic Contrasts: Interpretation of Anhydrite Veins: Conclusions Epilogue Acknowledgments ABSTRACT The Sheepbed mudstone forms the base of the strata examined by the Curiosity rover in Gale Crater on Mars, and is the first bona fide mudstone known on another planet. From images and associated data we propose a holistic interpretation of depositional regime, diagenesis, and burial history. A lake basin likely received sediment pulses from alluvial fans. Bed cross sections show mm-cm scale layering due to distal pulses of fluvial sediment injections (fine grained hyperpycnites), fall-out from river plumes, and some eolian supply. -

High-Resolution Stratigraphy and Lithology of an Outcrop Within the Grundy Formation (Pennsylvanian), Harlan County, Southeast Kentucky
Eastern Kentucky University Encompass Geosciences Undergraduate Theses Geosciences Spring 5-2020 High-resolution Stratigraphy and Lithology of an Outcrop within the Grundy Formation (Pennsylvanian), Harlan County, Southeast Kentucky Andrew Hensley Eastern Kentucky University Follow this and additional works at: https://encompass.eku.edu/geo_undergradtheses Recommended Citation Hensley, Andrew, "High-resolution Stratigraphy and Lithology of an Outcrop within the Grundy Formation (Pennsylvanian), Harlan County, Southeast Kentucky" (2020). Geosciences Undergraduate Theses. 7. https://encompass.eku.edu/geo_undergradtheses/7 This Restricted Access Thesis is brought to you for free and open access by the Geosciences at Encompass. It has been accepted for inclusion in Geosciences Undergraduate Theses by an authorized administrator of Encompass. For more information, please contact [email protected]. High-resolution Stratigraphy and Lithology of an Outcrop within the Grundy Formation (Pennsylvanian), Harlan County, Southeast Kentucky By Andrew Hensley Submitted to Walter S. Borowski Senior Thesis (GLY 499) May 2020 i TABLE OF CONTENTS Abstract……………………………………………………………………………………… 1 Introduction………………………………………………………………………….…….... 2 Methods……………………………………………………………………………………... 2 Results………………………………………………………………………………………..3 Coarsening-upward Sequence 1…………………..…………………………………5 Coarsening-upward Sequence 2…………………..…………………………………7 Coarsening-upward Sequence 3…………………..…………………………………9 Coarsening-upward Sequence 4…………………………………………………….11 Coarsening-upward Sequence -

SPE 131350 from Oil-Prone Source Rock to Gas-Producing Shale
SPE 131350 From Oil-Prone Source Rock to Gas-Producing Shale Reservoir – Geologic and Petrophysical Characterization of Unconventional Shale-Gas Reservoirs Q. R. Passey, K. M. Bohacs, W. L. Esch, R. Klimentidis, and S. Sinha, ExxonMobil Upstream Research Co. Copyright 2010, Society of Petroleum Engineers This paper was prepared for presentation at the CPS/SPE International Oil & Gas Conference and Exhibition in China held in Beijing, China, 8–10 June 2010. This paper was selected for presentation by a CPS/SPE program committee following review of information contained in an abstract submitted by the author(s). Contents of the paper have not been reviewed by the Society of Petroleum Engineers and are subject to correction by the author(s). The material does not necessarily reflect any position of the Society of Petroleum Engineers, its officers, or members. Electronic reproduction, distribution, or storage of any part of this paper without the written consent of the Society of Petroleum Engineers is prohibited. Permission to reproduce in print is restricted to an abstract of not more than 300 words; illustrations may not be copied. The abstract must contain conspicuous acknowledgment of SPE copyright. Abstract Many currently producing shale-gas reservoirs are overmature oil-prone source rocks. Through burial and heating these reservoirs evolve from organic-matter-rich mud deposited in marine, lacustrine, or swamp environments. Key characterization parameters are: total organic carbon (TOC), maturity level (vitrinite reflectance), mineralogy, thickness, and organic matter type. Hydrogen- to-carbon (HI) and oxygen-to-carbon (OI) ratios are used to classify organic matter that ranges from oil-prone algal and herbaceous to gas-prone woody/coaly material. -

Reservoir Characteristics and Logging Evaluation of Gas-Bearing
www.nature.com/scientificreports OPEN Reservoir characteristics and logging evaluation of gas−bearing mudstone in the south of North China Plain Liang Liu 1, Heping Pan1 ✉ , Zhenzhou Lin2 ✉ , Shihui Zhang1, Zhen Qin3, Jianwei Li4, Guoshu Huang1, Lei Wang4 & Dong Li1 Mudstone is very similar to shale except it lacks sheet bedding. Shale gas is widely concerned and successfully exploited commercially in the world, while gas-bearing mudstone is rarely paid attention. To evaluate the reservoir characteristics and exploitation potential of gas-bearing mudstone, a total of 127 mudstone samples from the Shanxi formation were tested by X-ray difraction (XRD), scanning electron microscope (SEM), gas content, etc., and the qualitative identifcation and quantitative evaluation of gas-bearing mudstone reservoirs were performed on four wells using the logging curve overlay method and reservoir parameter calculation equations. The results showed that: (1) the average total gas content of core measurement is 1.81 m3/t, and the total content of brittle minerals is 44.2%, which confrms that mudstones can also have good gas content and fracturing performance; (2) logging evaluation the average thickness of gas-bearing mudstone is 55.7 m, the average total gas content is 1.6 m3/t, and the average brittleness index is 38.1%, which indicates that the mudstone of Shanxi formation in the study area is generally gas-bearing and widely distributed. All the results reveal that gas-bearing mudstone with block bedding has the same exploitation potential as shale with sheet bedding,which deserves more attention. Mudstone and shale are very similar. Te diference is that the mudstone lacking the laminations of shale, which easily forms a block shape when it is broken, while shale has bedding structure, which splits easily into thin fat layers. -

Silicic Volcanism on Mars Evidenced by Tridymite in High-Sio2 Sedimentary Rock at Gale Crater Richard V
Silicic volcanism on Mars evidenced by tridymite in high-SiO2 sedimentary rock at Gale crater Richard V. Morrisa,1, David T. Vanimanb, David F. Blakec, Ralf Gellertd, Steve J. Chiperae, Elizabeth B. Rampef, Douglas W. Minga, Shaunna M. Morrisong, Robert T. Downsg, Allan H. Treimanh, Albert S. Yeni, John P. Grotzingerj,1, Cherie N. Achillesg, Thomas F. Bristowc, Joy A. Crispi, David J. Des Maraisc, Jack D. Farmerk, Kim V. Fendrichg, Jens Frydenvangl,m, Trevor G. Graffn, John-Michael Morookiani, Edward M. Stolperj, and Susanne P. Schwenzerh,o aNASA Johnson Space Center, Houston, TX 77058; bPlanetary Science Institute, Tucson, AZ 85719; cNASA Ames Research Center, Moffitt Field, CA 94035; dDepartment of Physics, University of Guelph, Guelph, ON, Canada N1G 2W1; eChesapeake Energy, Oklahoma City, OK 73118; fAerodyne Industries, Houston, TX 77058; gDepartment of Geosciences, University of Arizona, Tucson, AZ 85721; hLunar and Planetary Institute, Houston, TX 77058; iJet Propulsion Laboratory, California Institute of Technology, Pasadena, CA 91109; jDivision of Geological and Planetary Sciences, California Institute of Technology, Pasadena, CA 91125; kSchool of Earth and Space Exploration, Arizona State University, Tempe, AZ 85287; lLos Alamos National Laboratory, Los Alamos, NM 87545; mNiels Bohr Institute, University of Copenhagen, 2100 Copenhagen, Denmark; nJacobs, Houston, TX 77058; and oDepartment of Environment, Earth and Ecosystems, The Open University, Milton Keynes MK7 6AA, United Kingdom Contributed by John P. Grotzinger, May 5, 2016 (sent for review March 18, 2016); reviewed by Jon Blundy, Robert M. Hazen, and Harry Y. McSween) Tridymite, a low-pressure, high-temperature (>870 °C) SiO2 poly- (1), where three drill samples were analyzed by CheMin [Confi- morph, was detected in a drill sample of laminated mudstone (Buck- dence Hills, Mojave2, and Telegraph Peak (2)]. -

Constraining the Texture and Composition of Pore-Filling Cements at Gale Crater, Mars
46th Lunar and Planetary Science Conference (2015) 2234.pdf CONSTRAINING THE TEXTURE AND COMPOSITION OF PORE-FILLING CEMENTS AT GALE CRATER, MARS. K. L. Siebach1, J. P. Grotzinger1, S. M. McLennan2, J. A. Hurowitz2, D. W. Ming3, D. T. Vaniman4, E. B. Rampe5, D. L. Blaney6, L. C. Kah7, and the MSL Science Team. 1Division of Geological and Plane- tary Sciences, California Institute of Technology, Pasadena, CA, ([email protected]), 2Department of Geosci- ences, Stony Brook University, Stony Brook, NY, 3NASA JSC, Houston, TX, 4Planetary Science Institute, Tucson, AZ, 5Aerodyne Industries, Jacobs JETS Contract, NASA JSC, Houston, TX, 6NASA JPL, California Institute of Technology, Pasadena, CA, 7University of Tennessee, Department of Earth and Planetary Sciences, Knoxville, TN Introduction: The Mars Science Laboratory composition must be considered. Here, we attempt to (MSL) rover Curiosity has encountered a wide variety summarize the current understanding of the textural of sedimentary rocks deposited in fluvio-lacuestrine and compositional aspects of the cement across the sequences at the base of Gale Crater [1]. The presence rocks analyzed by Curiosity to this point. of sedimentary rocks requires that initial sediments Textural Observations: underwent diagenesis and were lithified. Lithification Macro-scale. Textural observations of the rocks involves sediment compaction, cementation, and re- Curiosity has surveyed begin at the macro scale. Cur- crystallization (or authigenic) processes. Analysis of rent holistic sedimentary models describe the formation the texture and composition of the cement can reveal of the traversed units using an aggradational delta the environmental conditions when the cements were model, which implies at least two sediment sources [8, deposited, enabling better understanding of early envi- 9]. -

Sedimentary Rocks and the Rock Cycle
Sedimentary Rocks and the Rock Cycle Designed to meet South Carolina Department of Education 2005 Science Academic Standards 1 Table of Contents What are Rocks? (slide 3) Major Rock Types (slide 4) (standard 3-3.1) The Rock Cycle (slide 5) Sedimentary Rocks (slide 6) Diagenesis (slide 7) Naming and Classifying Sedimentary Rocks (slide 8) Texture: Grain Size (slide 9), Sorting (slide 10) , and Rounding (slide 11) Texture and Weathering (slide 12) Field Identification (slide 13) Classifying Sedimentary Rocks (slide 14) Sedimentary Rocks: (slide 15) Clastic Sedimentary Rocks: Sandstone (16) , Siltstone (17), Shale (18), Mudstone (19) , Conglomerate (20), Breccia (21) , and Kaolin (22) Chemical Inorganic Sedimentary Rocks : Dolostone (23) and Evaporites (24) Chemical / Biochemical Sedimentary Rocks: Limestone (25) , Coral Reefs (26), Coquina and Chalk (27), Travertine (28) and Oolite (29) Chemical Organic Sedimentary Rocks : Coal (30), Chert (31): Flint, Jasper and Agate (32) Stratigraphy (slide 33) and Sedimentary Structures (slide 34 ) Sedimentary Rocks in South Carolina (slide 35) Sedimentary Rocks in the Landscape (slide 36) South Carolina Science Standards (slide 37) Resources and References (slide 38) 2 What are Rocks? Most rocks are an aggregate of one or more minerals and a few rocks are composed of non-mineral matter. There are three major rock types: 1. Igneous 2. Metamorphic 3. Sedimentary 3 Table of Contents Major Rock Types Igneous rocks are formed by the cooling of molten magma or lava near, at, or below the Earth’s surface. Sedimentary rocks are formed by the lithification of inorganic and organic sediments deposited at or near the Earth’s surface. Metamorphic rocks are formed when preexisting rocks are transformed into new rocks by elevated heat and pressure below the Earth’s surface. -

Organic Molecules in the Sheepbed Mudstone, Gale Crater, Mars
Volume 120 • Issue 3 • March 2015 • Pages 359–624 PUBLICATIONS Journal of Geophysical Research: Planets RESEARCH ARTICLE Organic molecules in the Sheepbed Mudstone, 10.1002/2014JE004737 Gale Crater, Mars Key Points: C. Freissinet1,2,D.P.Glavin1,P.R.Mahaffy1,K.E.Miller3,J.L.Eigenbrode1,R.E.Summons3,A.E.Brunner1,4, • First in situ evidence of nonterrestrial 5 6 7 1,8 9 1 6 10 organics in Martian surface sediments A. Buch ,C.Szopa ,P.D.ArcherJr. ,H.B.Franz ,S.K.Atreya , W. B. Brinckerhoff ,M.Cabane , P. Coll , 1 11 1 12,13 9 14 • Chlorinated hydrocarbons identified P. G. Conrad , D. J. Des Marais ,J.P.Dworkin ,A.G.Fairén , P. François ,J.P.Grotzinger , in the Sheepbed mudstone by SAM S. Kashyap1,8, I. L. ten Kate15, L. A. Leshin16, C. A. Malespin1,17, M. G. Martin1,18, F. J. Martin-Torres19,20, • Organics preserved in sample 1 21 22 1 1 12 exposed to ionizing radiation and A. C. McAdam , D. W. Ming , R. Navarro-González , A. A. Pavlov , B. D. Prats , S. W. Squyres , 23 1 24 7 25 oxidative condition A. Steele , J. C. Stern , D. Y. Sumner , B. Sutter , M.-P. Zorzano , and the MSL Science Team 1Solar System Exploration Division, NASA Goddard Space Flight Center, Greenbelt, Maryland, USA, 2NASA Postdoctoral Program, Oak Ridge Associated Universities, Oak Ridge, Tennessee, USA, 3Department of Earth, Atmospheric and Planetary Correspondence to: Sciences, Massachusetts Institute of Technology, Cambridge, Massachusetts, USA, 4Center for Research and Exploration C. Freissinet and P. R. Mahaffy, in Space Science & Technology, University of Maryland,