07 33880Rsj100818 53
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Districts of Ethiopia
Region District or Woredas Zone Remarks Afar Region Argobba Special Woreda -- Independent district/woredas Afar Region Afambo Zone 1 (Awsi Rasu) Afar Region Asayita Zone 1 (Awsi Rasu) Afar Region Chifra Zone 1 (Awsi Rasu) Afar Region Dubti Zone 1 (Awsi Rasu) Afar Region Elidar Zone 1 (Awsi Rasu) Afar Region Kori Zone 1 (Awsi Rasu) Afar Region Mille Zone 1 (Awsi Rasu) Afar Region Abala Zone 2 (Kilbet Rasu) Afar Region Afdera Zone 2 (Kilbet Rasu) Afar Region Berhale Zone 2 (Kilbet Rasu) Afar Region Dallol Zone 2 (Kilbet Rasu) Afar Region Erebti Zone 2 (Kilbet Rasu) Afar Region Koneba Zone 2 (Kilbet Rasu) Afar Region Megale Zone 2 (Kilbet Rasu) Afar Region Amibara Zone 3 (Gabi Rasu) Afar Region Awash Fentale Zone 3 (Gabi Rasu) Afar Region Bure Mudaytu Zone 3 (Gabi Rasu) Afar Region Dulecha Zone 3 (Gabi Rasu) Afar Region Gewane Zone 3 (Gabi Rasu) Afar Region Aura Zone 4 (Fantena Rasu) Afar Region Ewa Zone 4 (Fantena Rasu) Afar Region Gulina Zone 4 (Fantena Rasu) Afar Region Teru Zone 4 (Fantena Rasu) Afar Region Yalo Zone 4 (Fantena Rasu) Afar Region Dalifage (formerly known as Artuma) Zone 5 (Hari Rasu) Afar Region Dewe Zone 5 (Hari Rasu) Afar Region Hadele Ele (formerly known as Fursi) Zone 5 (Hari Rasu) Afar Region Simurobi Gele'alo Zone 5 (Hari Rasu) Afar Region Telalak Zone 5 (Hari Rasu) Amhara Region Achefer -- Defunct district/woredas Amhara Region Angolalla Terana Asagirt -- Defunct district/woredas Amhara Region Artuma Fursina Jile -- Defunct district/woredas Amhara Region Banja -- Defunct district/woredas Amhara Region Belessa -- -

19 Epidemiological Study of Bovine Trypanosomosis in Wenbera
Epidemiological Study Of Bovine Trypanosomosis In Wenbera District, Metekal Zone Of Benishagul Gumuz Regional State, Western Ethiopia [1] Dawit Tesfaye, [1] Tesfa Feleke and [2] Derara Birasa 1 National Tsetse flies and Trypanosomosis Control and Eradication Institute of Ethiopia, Assosa 2 Jimma University, College of Agriculture and Veterinary Medicine, School of Veterinary Medicine, P. O. Box 307, Jimma, Ethiopia Corresponding author: [email protected]; phone: +251910186937/+251910186937 Abstract: Cross-sectional study was conducted in Wanbera district of Benishangul-Gumuz Regional State, Western Ethiopia from February, 2019 to April 2019 to assess the prevalence of bovine trypanosomosis and association risk factors. During this survey, blood samples of 384 randomly selected cattle (Bosindicus) were examined using Buffy coat techniques. The packed cell volume (PCV) value of each animal was measured using hematocrit reader. Descriptive statistics was held to analyze the findings using STATA version 14.0 software packages. Chi square test was used to determine the association between different risk factors (age, sex, Body condition and location) and trypanosomosis infection. Out of 384 cattle examined, 8(2.08%) were found positive for trypanosomosis. The highest prevalence was revealed in Bagondy village 4 (50%) followed by Muz village 3(37.5%) and the lowest was recorded in Zamatiya village 1(12.5%). Trypanosome congolense (75%) was the most dominant trypanosome species identified followed by T. vivax (25%). The mean packed cell volume (PCV) value of infected animals was 17.92%±3.356 for trypanosome positive animals and 27.22%±2.748 for non-infected animals. Similarly, the highest prevalence (87.5%) of trypanosomosis infection was registered in animals with poor body condition score. -

Ethiopia Country Office Humanitarian Situation Report Includes Results from Tigray Response
Ethiopia Country Office Humanitarian Situation Report Includes results from Tigray Response © UNICEF Ethiopia/2021/Nahom Tesfaye Situation in Numbers Reporting Period: May 2021 12.5 million Highlights children in need of humanitarian assistance (HNO 2021) In May, 56,354 new medical consultations were conducted in Afar, Somali and Tigray regions through the 79 UNICEF- supported Mobile Health and Nutrition Teams (MHNTs), 23.5 million 11,692 of these in Tigray through the 30 active MHNTs. people in need UNICEF reached 412,647 people in May and 2,881,630 (HNO 2021) people between January to May 2021 throughout Ethiopia with safe water for drinking, cooking, and personal hygiene 2 through the rehabilitation of non-functional water systems, 3.6 million water treatment, and water trucking; of these, 1,228,921 were internally displaced people (DTM, in Tigray 2021) Since the beginning of the Tigray crisis, UNICEF has delivered 2,352 metric tons of multi-sectoral supplies to nine 806,541 partners (including Regional Bureaus) working in the region, valued at US$ 4.6 million. registered refugees (UNHCR,31 May 2021) In May, UNICEF supported the treatment of 38,032 under 5 children with Severe Acutely Malnutrition (SAM) in Ethiopia (1,723 in Tigray); 40.6 per cent of these were in Oromia, 20.7 per cent in Somali, 15.4 percent in SNNP/Sidama, 12.7 percent in Amhara and 4.5 per cent in Tigray. A total of UNICEF Revised HAC Appeal 152,413 children in the country have been treated for SAM between January – April 2021 with UNICEF direct support 2021 -

Running Head: COMMUNITY RESPONSE to OVC
Community Response to OVC…1 Running Head: COMMUNITY RESPONSE TO OVC Community Response to Provision of Care and Support for Orphans and Vulnerable Children, Constraints, Challenges and Opportunities: The Case of Chagni Town, Guangua Woreda Yohannes Mekuriaw Addis Ababa University A Thesis Submitted to the Research and Graduate Programs of Addis Ababa University in Partial Fulfillment of the Requirements for the Degree of Master of Social Work (MSW) Advisor: Professor Alice Johnson Butterfield June 2006 Addis Ababa, Ethiopia Community Response to OVC…2 Addis Ababa University Research and Graduate Program Community Response to Provision of Care and Support for Orphans and Vulnerable Children, Constraints, Challenges and Opportunities: The Case of Chagni Town, Guangua Woreda Yohannes Mekuriaw Graduate School Of Social Work Approved By Examining Board Advisor____________________ Signature _______Date_______________________ Examiner________________ Signature _________ Date_______________ Community Response to OVC…3 DEDICATION This Thesis is dedicated to my deceased Mother w/ro Simegnesh Shitahun without which my Educational Career Development would have been impossible. Community Response to OVC…4 Acknowledgement My first gratitude and appreciation goes to my thesis advisor, Prof. Alice K. Johnson Butterfield who critically commented on my thesis proposal and the report of the findings. Prof. Nathan Linsk also deserves this acknowledgment for his invitation to join a small group discussion with graduate students of social work who were working their Thesis on HIV/AIDS, and for his constructive comments on data collection tools and methods before fieldwork. I also thank Addis Ababa University for the small grant it provided to conduct the research. My wife Dejiytinu, with my child Kiduse, and my sister Rahael also shares this acknowledgement for I have been gone from my family role sets as a husband, father and a brother because of the engagement with my education these past two years. -

The Socioecological Significance of Dispersed Farmland Trees in Northern Ethiopia
Colby College Digital Commons @ Colby Honors Theses Student Research 2016 Missing the Trees for the Forest: The Socioecological Significance of Dispersed Farmland Trees in Northern Ethiopia Jacob A. Wall Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Environmental Studies Commons, Geographic Information Sciences Commons, Natural Resources and Conservation Commons, Natural Resources Management and Policy Commons, Nature and Society Relations Commons, Other Environmental Sciences Commons, Remote Sensing Commons, Spatial Science Commons, and the Sustainability Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Wall, Jacob A., "Missing the Trees for the Forest: The Socioecological Significance of Dispersed Farmland Trees in Northern Ethiopia" (2016). Honors Theses. Paper 950. https://digitalcommons.colby.edu/honorstheses/950 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Missing the Trees for the Forest: The Socioecological Significance of Dispersed Farmland Trees in Northern Ethiopia Jacob A. Wall Environmental Studies Program Colby College Waterville, Maine May 16, 2016 A thesis submitted to the faculty of the Environmental Studies Program in partial fulfillment of the graduation requirements for the Degree of Bachelor of Arts with honors in Environmental Studies. ________________________ _______________________ ____________________ Travis W. Reynolds, Advisor Manny Gimond, Reader Bruce Rueger, Reader i Copyright © 2016 by the Environmental Studies Program, Colby College. -

Modeling Malaria Cases Associated with Environmental Risk Factors in Ethiopia Using Geographically Weighted Regression
MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING GEOGRAPHICALLY WEIGHTED REGRESSION Berhanu Berga Dadi i MODELING MALARIA CASES ASSOCIATED WITH ENVIRONMENTAL RISK FACTORS IN ETHIOPIA USING THE GEOGRAPHICALLY WEIGHTED REGRESSION MODEL, 2015-2016 Dissertation supervised by Dr.Jorge Mateu Mahiques,PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain Ana Cristina Costa, PhD Professor, Nova Information Management School University of Nova Lisbon, Portugal Pablo Juan Verdoy, PhD Professor, Department of Mathematics University of Jaume I Castellon, Spain March 2020 ii DECLARATION OF ORIGINALITY I declare that the work described in this document is my own and not from someone else. All the assistance I have received from other people is duly acknowledged, and all the sources (published or not published) referenced. This work has not been previously evaluated or submitted to the University of Jaume I Castellon, Spain, or elsewhere. Castellon, 30th Feburaury 2020 Berhanu Berga Dadi iii Acknowledgments Before and above anything, I want to thank our Lord Jesus Christ, Son of GOD, for his blessing and protection to all of us to live. I want to thank also all consortium of Erasmus Mundus Master's program in Geospatial Technologies for their financial and material support during all period of my study. Grateful acknowledgment expressed to Supervisors: Prof.Dr.Jorge Mateu Mahiques, Universitat Jaume I(UJI), Prof.Dr.Ana Cristina Costa, Universidade NOVA de Lisboa, and Prof.Dr.Pablo Juan Verdoy, Universitat Jaume I(UJI) for their immense support, outstanding guidance, encouragement and helpful comments throughout my thesis work. Finally, but not least, I would like to thank my lovely wife, Workababa Bekele, and beloved daughter Loise Berhanu and son Nethan Berhanu for their patience, inspiration, and understanding during the entire period of my study. -
Shelter/NFI Cluster Coordination Meeting (Federal)
Shelter/NFI Cluster Coordination Meeting (Federal) Date of Meeting April 09, 2021 Time 10:00 AM Minutes prepared by: Cluster Location Webinar chaired by Shelter Cluster Attendees IRC, SWAN, Samaritan's Purse, FIDO, DPO, People in Need (PIN), SCI, IOM, ANE, Dorcas Aid International, ASDEPO, NRC, IOM/RRF, SCA/HEKS-EPER, UNHCR, LWF, MCMDO, WVI, CRS, AIDRO, ZOA, FIDO, NDRMC, ADPC, Islamic Relief, ACAPS, USAID/BHA, CARE Agenda 1. Review of action points of previous meeting 2. Overall response and Pipeline update 3. The current humanitarian situation in country and expectation from ESNFI 4. ESNFI Situation Update and Response in Tigray 5. Emergency Response Mechanisms, how it works 6. HLP assessment in West Tigray Zone AOB 1st round of EHF Agenda and notes. Decisions, issues 1. Review of Action Action Point Further Action Point • Partners should communicate their planned activities to On-going the sub-national Cluster and the regional ECC before addressing the beneficiaries • Partners should report age/sex disaggregated data for On-going completed activities from the actual distribution list, not a calculation of beneficiaries using the average household size. On-going • Partners are encouraged to share sector-specific funding of the year 2021 to the FTS website. The cluster will share the FTS template with link On-going • Coordination with the sub-national Cluster highly encouraged for any response 2. Information Please refer to the Shelter Cluster Presentation for more detail Management Updates(Overall • Response - Overall: In the first quarter of 2021, the cluster through its partners reached response and 357,424 beneficiaries with ESNFI kits, NFI kits, cash for rent and construction of communal Pipeline update) and emergency shelters by considering females, children, and people with different vulnerability. -
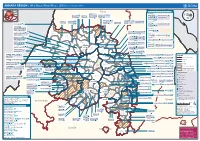
AMHARA REGION : Who Does What Where (3W) (As of 13 February 2013)
AMHARA REGION : Who Does What Where (3W) (as of 13 February 2013) Tigray Tigray Interventions/Projects at Woreda Level Afar Amhara ERCS: Lay Gayint: Beneshangul Gumu / Dire Dawa Plan Int.: Addis Ababa Hareri Save the fk Save the Save the df d/k/ CARE:f k Save the Children:f Gambela Save the Oromia Children: Children:f Children: Somali FHI: Welthungerhilfe: SNNPR j j Children:l lf/k / Oxfam GB:af ACF: ACF: Save the Save the af/k af/k Save the df Save the Save the Tach Gayint: Children:f Children: Children:fj Children:l Children: l FHI:l/k MSF Holand:f/ ! kj CARE: k Save the Children:f ! FHI:lf/k Oxfam GB: a Tselemt Save the Childrenf: j Addi Dessie Zuria: WVE: Arekay dlfk Tsegede ! Beyeda Concern:î l/ Mirab ! Concern:/ Welthungerhilfe:k Save the Children: Armacho f/k Debark Save the Children:fj Kelela: Welthungerhilfe: ! / Tach Abergele CRS: ak Save the Children:fj ! Armacho ! FHI: Save the l/k Save thef Dabat Janamora Legambo: Children:dfkj Children: ! Plan Int.:d/ j WVE: Concern: GOAL: Save the Children: dlfk Sahla k/ a / f ! ! Save the ! Lay Metema North Ziquala Children:fkj Armacho Wegera ACF: Save the Children: Tenta: ! k f Gonder ! Wag WVE: Plan Int.: / Concern: Save the dlfk Himra d k/ a WVE: ! Children: f Sekota GOAL: dlf Save the Children: Concern: Save the / ! Save: f/k Chilga ! a/ j East Children:f West ! Belesa FHI:l Save the Children:/ /k ! Gonder Belesa Dehana ! CRS: Welthungerhilfe:/ Dembia Zuria ! î Save thedf Gaz GOAL: Children: Quara ! / j CARE: WVE: Gibla ! l ! Save the Children: Welthungerhilfe: k d k/ Takusa dlfj k -

From Dust to Dollar Gold Mining and Trade in the Sudan–Ethiopia Borderland
From Dust to Dollar Gold mining and trade in the Sudan–Ethiopia borderland [Copy and paste completed cover here} Enrico Ille, Mohamed[Copy[Copy and and paste paste Salah completed completed andcover cover here} here} Tsegaye Birhanu image here, drop from 20p5 max height of box 42p0 From Dust to Dollar Gold mining and trade in the Sudan–Ethiopia borderland Enrico Ille, Mohamed Salah and Tsegaye Birhanu Cover image: Gold washers close to Qeissan, Sudan, 25 November 2019 © Mohamed Salah This report is a product of the X-Border Local Research Network, a component of the FCDO’s Cross-Border Conflict Evidence, Policy and Trends (XCEPT) programme, funded by UK aid from the UK government. XCEPT brings together leading experts to examine conflict-affected borderlands, how conflicts connect across borders, and the factors that shape violent and peaceful behaviour. The X-Border Local Research Network carries out research to better understand the causes and impacts of conflict in border areas and their international dimensions. It supports more effective policymaking and development programming and builds the skills of local partners. The views expressed do not necessarily reflect the UK government’s official policies. The Rift Valley Institute works in Eastern and Central Africa to bring local knowledge to bear on social, political and economic development. Copyright © Rift Valley Institute 2021. This work is published under a Creative Commons Attribution-NonCommercial-NoDerivatives License (CC BY-NC-ND 4.0) RIFT VALLEY INSTITUTE REPORT 2 Contents Executive summary 5 1. Introduction 7 Methodology 9 2. The Blue Nile–Benishangul-Gumuz borderland 12 The two borderland states 12 The international border 14 3. -

Commercialization of Teff Production in West North Ethiopia
Commercialization of Teff production in West North Ethiopia Habtamu Mossie ( [email protected] ) Injibara University of Ethiopia Dubale Abate Wolkite University Eden Kasse Injibara Minster of Finance and revenue Research Article Keywords: Double hurdle model, Market participation, Market orientation, Teff, Ethiopia Posted Date: August 16th, 2021 DOI: https://doi.org/10.21203/rs.3.rs-812219/v1 License: This work is licensed under a Creative Commons Attribution 4.0 International License. Read Full License Commercialization of Teff production in West North Ethiopia Habtamu Mossie1* Dubale Abate 2 Eden Kasse3 1*Department of Agricultural Economics, Inijbara University College of Agriculture, food and Climate Injibara, Ethiopia 2Department of Agribusiness and value chain management, Wolkite University, Wolkite, Ethiopia 3Eden Kasse Department of Agricultural Economics, Injibara Minster of Finance and revenue Corresponding author E-mail: habtamu. [email protected] ABSTRACT Background: Teff is only cereal crop Ethiopia’s in terms of production, acreage, and the number of farm holdings. It is one of the staples crops produced in the study area. However, the farm productivity, commercialization and level of intensity per hectare is low compared to the other cereals , Despite, smallholder farmers are not enough to participate in the teff market so the commercialization level is very low due to different factors. so, the study aimed to analyze determinants of smallholder farmer’s teff commercialization in west north, Ethiopia. Methods; A three-stage sampling procedure was used to take the sample respondents, 190 smallholder teff producers were selected to collect primary data through semi-structures questionnaires. Combinations of data analysis methods such as descriptive statistics and econometrics model (double hurdle) were used. -

The Effects of Lime on Acid Properties of Soil and on Faba Bean Yield in Banja District
American Scientific Research Journal for Engineering, Technology, and Sciences (ASRJETS) ISSN (Print) 2313-4410, ISSN (Online) 2313-4402 © Global Society of Scientific Research and Researchers http://asrjetsjournal.org/ 1. The Effects of Lime on Acid Properties of Soil and on Faba Bean Yield in Banja District. The Case of Sankit Lideta, Awi Zone Amhara Regional State, Ethiopia Ayalew Muluye Melsew* Begimeder College of Teachers Education, Po.Box-20, Debra Tabor, Ethiopia Email : [email protected] Abstracts This study was conducted in the known acid soil area of Ethiopia Awi zone which is found in the Amhara regional state. Five levels of lime introduced (0, 1150kg/ha, 2300kg/ha, 3450kg/ha, 4600kg/ha) on the faba bean productivity in the acidic soils. This experiment was arranged in a factorial experiment using randomized complete block design (RCBD) with three replications and 39 .13kg/ha Urea and100kg/ha TSP had been used as the source of N and P, respectively. Crop data such as plant height, biomass yield and grain yield , 50% flowering ,50% maturity,95% maturity, number of pods/plant ,number of seeds /pod ,number of seeds /plant ,lodging ,stand count at emerge and harvest collected and analyzed using SAS Software version 9. The result of this study indicated that 4600kg/ha, lime brought significantly higher result than the control. As lime level increased from the 0 to 4600kg/ha, grain yield, 50% flowering, biomass yield, number of pods /plant, number of seeds/plant increased. However, as lime level increased the number of seeds/ pod, stand count at emerges, stand count at harvest, 50%maturity, 95%maturity did not bring significant change. -

Phenotypic Characterization of Indigenous Chicken Ecotypes in Awi Zone, Ethiopia
Ecology and Evolutionary Biology 2020; 5(4): 131-139 http://www.sciencepublishinggroup.com/j/eeb doi: 10.11648/j.eeb.20200504.13 ISSN: 2575-3789 (Print); ISSN: 2575-3762 (Online) Phenotypic Characterization of Indigenous Chicken Ecotypes in Awi Zone, Ethiopia Andualem Yihun 1, Manzoor Ahmed Kirmani 2, Meseret Molla 3 1Department of Animal Science, College of Agriculture, Oda Bultum University, Chiro, Ethiopia 2Department of Animal Science, College of Agriculture and Veterinary Medicine, Jimma University, Jimma, Ethiopia 3Department of Animal Science, College of Agriculture and Natural Resource, University of Gonder, Gonder, Ethiopia Email address: To cite this article: Andualem Yihun, Manzoor Ahmed Kirmani, Meseret Molla. Phenotypic Characterization of Indigenous Chicken Ecotypes in Awi Zone, Ethiopia. Ecology and Evolutionary Biology . Vol. 5, No. 4, 2020, pp. 131-139. doi: 10.11648/j.eeb.20200504.13 Received : October 2, 2020; Accepted : October 21, 2020; Published : November 4, 2020 Abstract: The study was conducted in three districts of Awi zone in Amhara region, with the aim to characterize and identify the phenotypic variation of indigenous chicken ecotypes. A total of 720 indigenous chicken ecotypes were (504) females and (216) males from the whole districts) to describe qualitative and quantitative traits. Local chicken were mostly normally feathered and large phenotypic variability among ecotypes was observed for plumage color. A many plumage colors were identified in all districts in which Red in high-land and mid-land and Gebsima (grayish) colours in low-land were the predominant color of the study area beside a large diversity. The average body weight of local chickens in high-land, mid-land and low-land agro-ecologies were 1.476, 1.75 and 1.71kg respectively, while the respective values for mature cocks and hens were 1.78 and 1.51kg.