A. Kumbang Dewasa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dimensional Limits for Arthropod Eyes with Superposition Optics
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Vision Research 44 (2004) 2213–2223 www.elsevier.com/locate/visres Dimensional limits for arthropod eyes with superposition optics Victor Benno Meyer-Rochow a,*,Jozsef Gal b a School of Engineering and Sciences, International University Bremen (IUB), Campus Ring 6, Research II, D-28759 Bremen, Germany b Institute of Plant Biology, Biological Research Centre, Hungarian Academy of Sciences, Temesvari krt. 62, H-6701 Szeged, Hungary Received 10 December 2003; received in revised form 16 April 2004 Abstract An essential feature of the superposition type of compound eye is the presence of a wide zone, which is transparent and devoid of pigment and interposed between the distal array of dioptric elements and the proximally placed photoreceptive layer. Parallel rays, collected by many lenses, must (through reflection or refraction) cross this transparent clear-zone in such a way that they become focused on one receptor. Superposition depends mostly on diameter and curvature of the cornea, size and shape of the crystalline cone, lens cylinder properties of cornea and cone, dimensions of the receptor cells, and width of the clear-zone. We examined the role of the latter by geometrical, geometric-optical, and anatomical measurements and concluded that a minimal size exists, below which effective superposition can no longer occur. For an eye of a given size, it is not possible to increase the width of the clear-zone cz ¼ dcz=R1 and decrease R2 (i.e., the radius of curvature of the distal retinal surface) and/or c ¼ dc=R1 without reaching a limit. -

Complete Mitochondrial Genome of Prismognathus Prossi (Coleoptera: Lucanidae) with Phylogenetic Implications
© Entomologica Fennica. 10 July 2019 Complete mitochondrial genome of Prismognathus prossi (Coleoptera: Lucanidae) with phylogenetic implications Jing Liu, Yuyan Cao, Shiju Zhou, Yongjing Chen & Xia Wan* Liu, J., Cao, Y.Y.,Zhou, S.J., Chen, Y.J. & Wan, X. 2019: Complete mitochondrial genome of Prismognathus prossi (Coleoptera: Lucanidae) with phylogenetic im- plications. — Entomol. Fennica 30: 90–96. https://doi.org/10.33338/ef.82927 The complete mitochondrial genome of a Chinese stag beetle, Prismognathus prossi, was generated using the Illumina next-generation sequencing. The mito- genome sequence is 15,984 bp in length, the nucleotide composition is A 36.6%, C 17.5%, T 34.3% and G 11.6% with the AT-content of 70.9%. The sequence has similar features with other reported insect mitogenomes, consisting of 13 protein- coding genes (PCGs), 22 transfer RNAgenes, two ribosomal RNAs and a control region. All of the protein-coding genes start with the typical ATN initiation codon except for COI. Maximum Likelihood (ML) and Bayesian Inference (BI) indi- cated that P. prossi share an affinity with Lucanus mazama, Lucanus fortunei and Cyclommatus vitalisi. J. Liu, Y.Y. Cao, S.J. Zhou, Y.J. Chen & X. Wan, Department of Ecology, School of Resources and Engineering, Anhui University, Anhui, Hefei, 23000, China. E-mails: Jing Liu: [email protected]; Yuyan Cao: 1210252162@163. com; Shiju Zhou: [email protected]; Yongjing Chen: cyj1452394259 @163.com: Xia Wan (* corresponding author): [email protected] Received 28 February 2018, accepted 13 September 2018 1. Introduction (Sheffield et al. 2009, Kim et al. 2013, Wu et al. -

Divergence in Gut Bacterial Community Among Life Stages of the Rainbow Stag Beetle Phalacrognathus Muelleri (Coleoptera: Lucanidae)
insects Article Divergence in Gut Bacterial Community Among Life Stages of the Rainbow Stag Beetle Phalacrognathus muelleri (Coleoptera: Lucanidae) 1,2, 1,2, 1,2, Miaomiao Wang y, Xingjia Xiang y and Xia Wan * 1 School of Resources and Environmental Engineering, Anhui University, Hefei 230601, China; [email protected] (M.W.); [email protected] (X.X.) 2 Anhui Province Key Laboratory of Wetland Ecosystem Protection and Restoration, Hefei 230601, China * Correspondence: [email protected] These authors contributed equally to this article. y Received: 19 September 2020; Accepted: 17 October 2020; Published: 21 October 2020 Simple Summary: Phalacrognathus muelleri is naturally distributed in Queensland (Australia) and New Guinea, and this species can be successfully bred under artificial conditions. In this study, we compared gut bacterial community structure among different life stages. There were dramatic shifts in gut bacterial community structure between larvae and adults, which was probably shaped by their diet. The significant differences between early instar and final instars larvae suggested that certain life stages are associated with a defined gut bacterial community. Our results contribute to a better understanding of the potential role of gut microbiota in a host’s growth and development, and the data will benefit stag beetle conservation in artificial feeding conditions. Abstract: Although stag beetles are popular saprophytic insects, there are few studies about their gut bacterial community. This study focused on the gut bacterial community structure of the rainbow stag beetle (i.e., Phalacrognathus muelleri) in its larvae (three instars) and adult stages, using high throughput sequencing (Illumina Miseq). Our aim was to compare the gut bacterial community structure among different life stages. -

The Mystery of the Lesser Stag Beetle Dorcus Parallelipipedus (L.) (Coleoptera: Lucanidae) Mycangium Yeasts by Masahiko Tanahashi 1 and Maria Fremlin *2
146 Bulletin of the Amateur Entomologists' Society The mystery of the lesser stag beetle Dorcus parallelipipedus (L.) (Coleoptera: Lucanidae) mycangium yeasts by Masahiko Tanahashi 1 and Maria Fremlin *2 1 National Institute of Advanced Industrial Science and Technology (AIST) Central 6, Tsukuba, Ibaraki 305-8566, J apan 2 25 Ireton Rd, Colch ester, Essex, CO3 3AT, UK - E-mail: [email protected] (Correspondence) Introduction The mycangium is a microbe-storage organ present in several wood-feeding invertebrates; for example, it has been known for some time that bark and ambrosia beetles, and even leaf-roller weevils carry symbiotic fungi in special structures on their bodies (Beaver, 1989; Kobayashi et al , 2008). However, this organ has only been discovered recently in stag beetles (Tanahashi et al. , 2010) and in a certain lizard beetle ( Toki et al ., 2012 ), probably because it is not an external structure. In their case, the mycangium is an ovipositor- associated organ, that is, an exoskeletal organ normally folded and hidden under the last tergal plate of the female’s abdomen. Remarkably, this organ is clearly represented, albeit without a description, in Franciscolo’s drawings of the dorsal view of the female reproductive system for a couple of Lucanidae species: Lucanus cervus and L. tetraodon (1997). However, he opted for ventral views of all the other species, including Dorcus parallelipipedus , in which case the mycangium is obscured by the reproductive organs. Since the author is no longer alive and the book is currently out of print, we reproduce below one of his diagrams with the mycangium marked by us (Figure 1). -

The Early Evolution of Biting–Chewing Performance in Hexapoda
Chapter 6 The Early Evolution of Biting–Chewing Performance in Hexapoda Alexander Blanke Abstract Insects show a plethora of different mandible shapes. It was advocated that these mandible shapes are mainly a function of different feeding habits. This hypothesis was tested on a larger sampling of non-holometabolan biting–chewing insects with additional tests to understand the interplay of mandible function, feeding guild, and phylogeny. The results show that at the studied systematic level, variation in mandible biting–chewing effectivity is regulated to a large extent by phylogenetic history and the configuration of the mandible joints rather than the food preference of a given taxon. Additionally, lineages with multiple mandibular joints such as primary wingless hexapods show a wider functional space occupation of mandibular effectivity than dicondylic insects (¼ silverfish + winged insects) at significantly different evolutionary rates. The evolution and occupation of a compa- rably narrow functional performance space of dicondylic insects is surprising given the low effectivity values of this food uptake solution. Possible reasons for this relative evolutionary “stasis” are discussed. 6.1 Introduction Insecta sensu lato (¼ Hexapoda) display a high diversity of mouthpart shapes within the early evolved lineages which started to radiate approximately 479 million years ago (Misof et al. 2014). These shape changes were described qualitatively and were often stated to relate mainly to the type of food consumed (Yuasa 1920; Isely 1944; Evans and Forsythe 1985; Chapman and de Boer 1995). To the knowledge of the author, this and related statements regarding mouthpart mechanics being shaped by functional demands have never been tested in a quantitative framework. -

2008/2009 Annual Report Australian
The Hon. Nathan Rees, MP Premier and Minister for the Arts Sir, In accordance with the provisions of the Annual Reports (Statutory Bodies) Act 1984 and the Public Finance and Audit Act 1983 we have pleasure in submitting this report of the activities of the Australian Museum Trust for the financial year ended 30 June 2009 for presentation to Parliament. On behalf of the Australian Museum Trust, Brian Sherman, AM President of the Trust Frank Howarth Secretary of the Trust Australian Museum Annual Report 2008–2009 iii MINISTER AUSTRALIAN MUSEUM The Hon. Nathan Rees, MP 6 College Street Sydney NSW 2010 Premier and Minister for the Arts Open daily 9.30 am – 5.00 pm (except 25 December) GOVERNANCE t 02 9320 6000 f 02 9320 6050 The Museum is governed by a Trust [email protected] established under the Australian Museum www.australianmuseum.net.au Trust Act 1975. The Trust currently has eleven members, one of whom must have ADMISSION CHARGES knowledge of, or experience in, science, one of whom must have knowledge of, or General Museum entry experience in, education and one of whom Adult $12 must have knowledge of, or experience Child (5 –15 years) $6 in, Australian Indigenous culture. Trustees are appointed by the Governor on the Concession $8 recommendation of the Minister for a Family (one adult, two children) $18 term of up to three years. Trustees may Family (two adults, two children) $30 hold no more than three terms. Vacancies Each additional child $3 may be filled by the Governor on the recommendation of the Minister. -

Phylogeography and DNA-Based Species
RESEARCH ARTICLE Phylogeography and DNA-based species delimitation provide insight into the taxonomy of the polymorphic rose chafer Protaetia (Potosia) cuprea species complex (Coleoptera: Scarabaeidae: Cetoniinae) in the Western Palearctic a1111111111 Dominik VondraÂček1☯*, Aneta Fuchsova 1³, Dirk Ahrens2³, David KraÂl1³, Petr SÏ Âõpek1☯ a1111111111 a1111111111 1 Department of Zoology, Faculty of Science, Charles University, Prague, Czech Republic, 2 Department of Arthropoda, Zoologisches Forschungsmuseum Alexander Koenig, Bonn, Germany a1111111111 a1111111111 ☯ These authors contributed equally to this work. ³ These authors also contributed equally to this work. * [email protected] OPEN ACCESS Abstract Citation: VondraÂček D, Fuchsova A, Ahrens D, KraÂl D, SÏÂõpek P (2018) Phylogeography and DNA-based The development of modern methods of species delimitation, unified under the ªintegrated species delimitation provide insight into the taxonomyº approach, allows a critical examination and re-evaluation of complex taxonomic taxonomy of the polymorphic rose chafer Protaetia groups. The rose chafer Protaetia (Potosia) cuprea is a highly polymorphic species group (Potosia) cuprea species complex (Coleoptera: Scarabaeidae: Cetoniinae) in the Western with a large distribution range. Despite its overall commonness, its taxonomy is unclear and Palearctic. PLoS ONE 13(2): e0192349. https://doi. subject to conflicting hypotheses, most of which largely fail to account for its evolutionary org/10.1371/journal.pone.0192349 history. Based on the sequences of two mitochondrial markers from 65 individuals collected Editor: Bi-Song Yue, Sichuan University, CHINA across the species range, and a detailed analysis of morphological characters including a Received: November 6, 2017 geometric morphometry approach, we infer the evolutionary history and phylogeography of the P. cuprea species complex. -

1 Evolution of Sexual Development and Sexual Dimorphism in Insects 1
1 Evolution of sexual development and sexual dimorphism in insects 2 3 Ben R. Hopkins1* & Artyom Kopp1 4 5 1. Department of Evolution and Ecology, University of California – Davis, Davis, USA 6 7 * Corresponding author 8 9 Corresponding author email: [email protected] 10 11 12 13 14 Abstract 15 Most animal species consist of two distinct sexes. At the morphological, physiological, and 16 behavioural levels the differences between males and females are numerous and dramatic, yet 17 at the genomic level they are often slight or absent. This disconnect is overcome because simple 18 genetic differences or environmental signals are able to direct the sex-specific expression of a 19 shared genome. A canonical picture of how this process works in insects emerged from decades 20 of work on Drosophila. But recent years have seen an explosion of molecular-genetic and 21 developmental work on a broad range of insects. Drawing these studies together, we describe 22 the evolution of sexual dimorphism from a comparative perspective and argue that insect sex 23 determination and differentiation systems are composites of rapidly evolving and highly 24 conserved elements. 25 1 26 Introduction 27 Anisogamy is the definitive sex difference. The bimodality in gamete size it describes 28 represents the starting point of a cascade of evolutionary pressures that have generated 29 remarkable divergence in the morphology, physiology, and behaviour of the sexes [1]. But 30 sexual dimorphism presents a paradox: how can a genome largely shared between the sexes 31 give rise to such different forms? A powerful resolution is via sex-specific expression of shared 32 genes. -
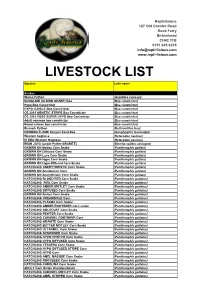
Livestock List
Repti-lisious 187 Old Chester Road Rock Ferry Birkenhead CH42 3TB 0151 645 6235 [email protected] www.repti-lisious.com LIVESTOCK LIST Species Latin name Snakes Column1 Woma Python (Aspidites ramsayi) SUNGLOW (ALBINO SHARP) Boa (Boa constrictor) Hypo Boa Constrictor (Boa constrictor) HYPO JUNGLE Boa Constrictor (Boa constrictor) CB 2015 GENETIC STRIPE Boa Constrictor (Boa constrictor) CB 2015 POSS SUPER HYPO Boa Constrictor (Boa constrictor) Adult common boa constrictor (Boa constrictor) Blood salmon boa constrictor (Boa constrictor) Bismark Python (Bothrochilus boa) DODOMA FLAME Kenyan Sand Boa (Gonglyophis loveredgei) Western hognose (Heterodon nasicus) HI RED Western Hognose (Heterodon nasicus) IRIAN JAYA Carpet Py(het GRANITE) (Morelia spilota variagata) GROWN ON Motley Corn Snake (Pantherophis guttata) GROWN ON Diffused Corn Snake (Pantherophis guttata) GROWN ON Lava Corn Snake (Pantherophis guttata) GROWN ON Hypo Corn Snake (Pantherophis guttata) GROWN ON Hypo Diffused Corn Snake (Pantherophis guttata) HATCHLING ANERYTHRISTIC Corn Snake (Pantherophis guttata) GROWN ON Amelanistic Corn (Pantherophis guttata) GROWN ON Anerythristic Corn Snake (Pantherophis guttata) HATCHLING BLOOD RED Corn Snake (Pantherophis guttatus) HATCHLING FIRE Corn Snake (Pantherophis guttatus) HATCHLING AMBER MOTLEY Corn Snake (Pantherophis guttatus) HATCHLING DIFFUSED Corn Snake (Pantherophis guttatus) GROWN ON Amber Corn Snake (Pantherophis guttatus) HATCHLING CREAMSICLE Corn (Pantherophis guttatus) HATCHLING PLASMA Corn Snake (Pantherophis guttatus) -

The Evolution of Animal Weapons
The Evolution of Animal Weapons Douglas J. Emlen Division of Biological Sciences, The University of Montana, Missoula, Montana 59812; email: [email protected] Annu. Rev. Ecol. Evol. Syst. 2008. 39:387-413 Key Words First published online as a Review in Advance on animal diversity, sexual selection, male competition, horns, antlers, tusks September 2, 2008 The Annual Review of Ecology, Evolution, and Abstract Systematics is online at ecolsys.annualreviews.org Males in many species invest substantially in structures that are used in com- This article's doi: bat with rivals over access to females. These weapons can attain extreme 10.1146/annurev.ecolsys.39.110707.173 502 proportions and have diversified in form repeatedly. I review empirical lit- Copyright © 2008 by Annual Reviews. erature on the function and evolution of sexually selected weapons to clarify All rights reserved important unanswered questions for future research. Despite their many 1543-592X/08/1201-0387$20.00 shapes and sizes, and the multitude of habitats within which they function, animal weapons share many properties: They evolve when males are able to defend spatially restricted critical resources, they are typically the most variable morphological structures of these species, and this variation hon- estly reflects among-individual differences in body size or quality. What is not clear is how, or why, these weapons diverge in form. The potential for male competition to drive rapid divergence in weapon morphology remains one of the most exciting and understudied topics in sexual selection research today. 3*7 INTRODUCTION Sexual selection is credited with the evolution of nature's most extravagant structures, and these include showy male adornments that are attractive to females (ornaments) and an arsenal of outgrowths that function in male-male combat (weapons) (Darwin 1871). -

WORLD LIST of EDIBLE INSECTS 2015 (Yde Jongema) WAGENINGEN UNIVERSITY PAGE 1
WORLD LIST OF EDIBLE INSECTS 2015 (Yde Jongema) WAGENINGEN UNIVERSITY PAGE 1 Genus Species Family Order Common names Faunar Distribution & References Remarks life Epeira syn nigra Vinson Nephilidae Araneae Afregion Madagascar (Decary, 1937) Nephilia inaurata stages (Walck.) Nephila inaurata (Walckenaer) Nephilidae Araneae Afr Madagascar (Decary, 1937) Epeira nigra Vinson syn Nephila madagscariensis Vinson Nephilidae Araneae Afr Madagascar (Decary, 1937) Araneae gen. Araneae Afr South Africa Gambia (Bodenheimer 1951) Bostrichidae gen. Bostrichidae Col Afr Congo (DeFoliart 2002) larva Chrysobothris fatalis Harold Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) larva Lampetis wellmani (Kerremans) Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) syn Psiloptera larva wellmani Lampetis sp. Buprestidae Col jewel beetle Afr Togo (Tchibozo 2015) as Psiloptera in Tchibozo but this is Neotropical Psiloptera syn wellmani Kerremans Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) Psiloptera is larva Neotropicalsee Lampetis wellmani (Kerremans) Steraspis amplipennis (Fahr.) Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) larva Sternocera castanea (Olivier) Buprestidae Col jewel beetle Afr Benin (Riggi et al 2013) Burkina Faso (Tchinbozo 2015) Sternocera feldspathica White Buprestidae Col jewel beetle Afr Angola (DeFoliart 2002) adult Sternocera funebris Boheman syn Buprestidae Col jewel beetle Afr Zimbabwe (Chavanduka, 1976; Gelfand, 1971) see S. orissa adult Sternocera interrupta (Olivier) Buprestidae Col jewel beetle Afr Benin (Riggi et al 2013) Cameroun (Seignobos et al., 1996) Burkina Faso (Tchimbozo 2015) Sternocera orissa Buquet Buprestidae Col jewel beetle Afr Botswana (Nonaka, 1996), South Africa (Bodenheimer, 1951; syn S. funebris adult Quin, 1959), Zimbabwe (Chavanduka, 1976; Gelfand, 1971; Dube et al 2013) Scarites sp. Carabidae Col ground beetle Afr Angola (Bergier, 1941), Madagascar (Decary, 1937) larva Acanthophorus confinis Laporte de Cast. -

A Multilocus Assessment Reveals Two New Synonymies for East Asian
ZooKeys 1021: 65–79 (2021) A peer-reviewed open-access journal doi: 10.3897/zookeys.1021.58832 RESEARCH ARTICLE https://zookeys.pensoft.net Launched to accelerate biodiversity research A multilocus assessment reveals two new synonymies for East Asian Cyclommatus stag beetles (Coleoptera, Lucanidae) Jiao Jiao Yuan1,2, Dan Chen1,2, Xia Wan1,2 1 Department of Ecology, School of Resources and Engineering, Anhui University, 111 Jiulong Rd., Hefei 230601, China 2 Anhui Province Key Laboratory of Wetland Ecosystem Protection and Restoration, Hefei 230601, China Corresponding author: Xia Wan ([email protected]) Academic editor: A. Frolov | Received 26 September 2020 | Accepted 31 January 2021 | Published 2 March 2021 http://zoobank.org/23EF341F-3DAF-4D3C-8292-4AE87334E1B9 Citation: Yuan JJ, Chen D, Wan X (2021) A multilocus assessment reveals two new synonymies for East Asian Cyclommatus stag beetles (Coleoptera, Lucanidae). ZooKeys 1021: 65–79. https://doi.org/10.3897/zookeys.1021.58832 Abstract Cyclommatus scutellaris Möllenkamp, 1912, Cyclommatus elsae Kriesche, 1921 and Cyclommatus tam- daoensis Fujita, 2010 are East Asian stag beetle species with long-debated taxonomic relationships due to high intraspecific morphological variability. In this study, we applied multilocus phylogenetic analyses to reassess their relationships. Two mitochondrial genes (16S rDNA, COI) and two nuclear genes (28S rDNA, Wingless) were used to reconstruct the phylogeny through the Bayesian inference (BI) and Maxi- mum Likelihood (ML) methods. Both topologies supported two clades: the clade C. scutellaris was sister to the clade (C. elsae + C. tamdaoensis) with the subclade C. tamdaoensis embedded in the subclade C. elsae. The Kimura 2-parameter (K2P) genetic distance analysis yielded a low mean value (≤0.035) among the three taxa, which was well below the minimum mean value between other Cyclommatus species (≥0.122).