Convergence of Three Mangrove Species Towards Freeze‐Tolerant
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Coastal Scrub and Chaparral Bird Conservation Plan
The Coastal Scrub and Chaparral Bird Conservation Plan A Strategy for Protecting and Managing Coastal Scrub and Chaparral Habitats and Associated Birds in California A Project of California Partners in Flight and PRBO Conservation Science The Coastal Scrub and Chaparral Bird Conservation Plan A Strategy for Protecting and Managing Coastal Scrub and Chaparral Habitats and Associated Birds in California Version 2.0 2004 Conservation Plan Authors Grant Ballard, PRBO Conservation Science Mary K. Chase, PRBO Conservation Science Tom Gardali, PRBO Conservation Science Geoffrey R. Geupel, PRBO Conservation Science Tonya Haff, PRBO Conservation Science (Currently at Museum of Natural History Collections, Environmental Studies Dept., University of CA) Aaron Holmes, PRBO Conservation Science Diana Humple, PRBO Conservation Science John C. Lovio, Naval Facilities Engineering Command, U.S. Navy (Currently at TAIC, San Diego) Mike Lynes, PRBO Conservation Science (Currently at Hastings University) Sandy Scoggin, PRBO Conservation Science (Currently at San Francisco Bay Joint Venture) Christopher Solek, Cal Poly Ponoma (Currently at UC Berkeley) Diana Stralberg, PRBO Conservation Science Species Account Authors Completed Accounts Mountain Quail - Kirsten Winter, Cleveland National Forest. Greater Roadrunner - Pete Famolaro, Sweetwater Authority Water District. Coastal Cactus Wren - Laszlo Szijj and Chris Solek, Cal Poly Pomona. Wrentit - Geoff Geupel, Grant Ballard, and Mary K. Chase, PRBO Conservation Science. Gray Vireo - Kirsten Winter, Cleveland National Forest. Black-chinned Sparrow - Kirsten Winter, Cleveland National Forest. Costa's Hummingbird (coastal) - Kirsten Winter, Cleveland National Forest. Sage Sparrow - Barbara A. Carlson, UC-Riverside Reserve System, and Mary K. Chase. California Gnatcatcher - Patrick Mock, URS Consultants (San Diego). Accounts in Progress Rufous-crowned Sparrow - Scott Morrison, The Nature Conservancy (San Diego). -

Conservation Issues: California Chaparral
Author's personal copy Conservation Issues: California Chaparral RW Halsey, California Chaparral Institute, Escondido, CA, United States JE Keeley, U.S. Geological Survey, Three Rivers, CA, United States ã 2016 Elsevier Inc. All rights reserved. What Is Chaparral? 1 California Chaparral Biodiversity 1 Chaparral Community Types 1 Measuring Chaparral Biodiversity 4 Diversity Within Individual Plant Taxa 5 Faunal Diversity 5 Influence of Geology 7 Influence of Climate 7 Influence of Fire 8 Impact of Climate Change 10 Preserving Chaparral Biodiversity 10 References 10 What Is Chaparral? Chaparral is a diverse, sclerophyllous shrub-dominated plant community shaped by a Mediterranean-type climate (hot, dry summers and mild, wet winters), a complex mixture of relatively young soils (Specht and Moll, 1983), and large, infrequent, high-intensity fires (30–150 year fire return interval) (Keeley and Zedler, 2009; Keeley et al., 2004; Lombardo et al., 2009). Large expanses of dense chaparral vegetation cover coastal mesas, canyons, foothills, and mountain slopes throughout the California Floristic Province (Figure 1), southward into Baja California, and extending north into the Rogue River Valley of southwest Oregon. Disjunct patches of chaparral can also be found in central and southeastern Arizona and northern Mexico (Keeley, 2000). Along with the four other Mediterranean-type climate regions of the world with similar shrubland vegetation (Central Chile, Mediterranean Basin, South Africa, and southwestern Australia) (Table 1), California has been designated a biodiversity hot spot (Myers et al., 2000). Twenty-five designated locations in all, these hot spots have exceptional concentrations of endemic species that are undergoing exceptional loss of habitat (Myers et al., 2000; Rundel, 2004). -

Oak Woodlands and Chaparral
Oak Woodlands and Chaparral Aligning chaparral-associated bird needs with oak woodland restoration and fuel reduction in southwest Oregon and northern California Why conservation is needed Oak woodland habitat in southwest Oregon and northern California is comprised of a vegetation gradient that includes oak savanna, open oak woodlands with grass or chaparral understory, closed canopy oak woodlands, and oak conifer forests. Oak habitats are at risk due to development, encroachment of coniferous forest, invasion of exotic species, and lack of oak regeneration. Birds and other wildlife that depend on these ecosystems have been negatively affected by habitat threats. Photo by Jaime Stephens What is chaparral and why is it important for birds? “Chaparral” is a short, shrubby vegetation type that can be composed of a variety of plant species. In this region, chaparral habitat is often associated with oak woodlands. Chaparral is a natural part of oak habitats, but it also poses a risk of spreading severe fire which can put large, old oak trees at risk. Because oak woodlands are threatened by loss and degradation, management initiatives sometimes reduce chaparral to reduce the risk of high severity fire and promote a mix of low to moderate severity fire. Still, functioning oak woodland mosaics in southern Oregon need many types of vegetation cover, including patches of chaparral. Restoring and managing oak woodland ecosystems in this region requires learning how to best achieve a balanced vegetation composition that includes chaparral habitat components. KBO, in partnership with the Klamath Siskiyou Oak Network, has conducted studies to determine how we might best manage oak and chaparral habitat for bird species. -

The Chaparral Vegetation in Mexico Under Nonmediterranean Climate: the Convergence and Madrean-Tethyan Hypotheses Reconsidered1
American Journal of Botany 85(10): 1398±1408. 1998. THE CHAPARRAL VEGETATION IN MEXICO UNDER NONMEDITERRANEAN CLIMATE: THE CONVERGENCE AND MADREAN-TETHYAN HYPOTHESES RECONSIDERED1 ALFONSO VALIENTE-BANUET,2,4 NOEÂ FLORES-HERNAÂ NDEZ,2 MIGUEL VERDUÂ ,3 AND PATRICIA DAÂ VILA3 2Instituto de EcologõÂa, Universidad Nacional AutoÂnoma de MeÂxico, Apartado Postal 70±275, UNAM, 04510 MeÂxico, D.F.; and 3UBIPRO, ENEP-Iztacala, Universidad Nacional AutoÂnoma de MeÂxico, Apartado Postal 314, MeÂxico, 54090, Tlalnepantla, MeÂxico A comparative study between an unburned evergreen sclerophyllous vegetation located in south-central Mexico under a wet-summer climate, with mediterranean regions was conducted in order to re-analyze vegetation and plant characters claimed to converge under mediterranean climates. The comparison considered ¯oristic composition, plant-community struc- ture, and plant characters as adaptations to mediterranean climates and analyzed them by means of a correspondence analysis, considering a tropical spiny shrubland as the external group. We made a species register of the number of species that resprouted after a ®re occurred in 1995 and a distribution map of the evergreen sclerophyllous vegetation in Mexico (mexical) under nonmediterranean climates. The TehuacaÂn mexical does not differ from the evergreen sclerophyllous areas of Chile, California, Australia, and the Mediterranean Basin, according to a correspondence analysis, which ordinated the TehuacaÂn mexical closer to the mediter- ranean areas than to the external group. All the vegetation and ¯oristic characteristics of the mexical, as well as its distribution along the rain-shadowed mountain parts of Mexico, support its origin in the Madrean-Tethyan hypothesis of Axelrod. Therefore, these results allow to expand the convergence paradigm of the chaparral under an integrative view, in which a general trend to aridity might explain ¯oristic and adaptive patterns detected in these environments. -

Biodiversity and Management of the Madrean Archipelago
)I This file was created by scanning the printed publication. Errors identified by the software have been corrected; however, some errors may remain. A Classification System and Map of the Biotic Communities of North America David E. Brown, Frank Reichenbacher, and Susan E. Franson 1 Abstract.-Biotic communities (biomes) are regional plant and animal associations within recognizable zoogeographic and floristic provinces. Using the previous works and modified terminology of biologists, ecologists, and biogeographers, we have developed an hierarchical classification system for the world's biotic communities. In use by the Arid Ecosystems Resource Group of the Environmental Protection Agency's Environmental Monitoring and Assessment Program, the Arizona Game and Fish Department, and other Southwest agencies, this classification system is formulated on the limiting effects of moisture and temperature minima on the structure and composition of vegetation while recognizing specific plant and animal adaptations to regional environments. To illustrate the applicability of the classification system, the Environmental Protection Agency has funded the preparation of a 1: 10,000,000 color map depicting the major upland biotic communities of North America using an ecological color scheme that shows gradients in available plant moisture, heat, and cold. Digitized and computer compatible, this hierarchical system facilitates biotic inventory and assessment, the delineation and stratification of habitats, and the identification of natural areas in need of acquisition, Moreover, the various categories of the classification are statistically testable through the use of existing climatic data, and analysis of plant and animal distributions. Both the classification system and map are therefore of potential use to those interested in preserving biotic diversity. -

Climate Zones and Biomes
Geography Climate Zones and Biomes Pupil Workbook Year 3, Unit 4 Name: Formative Assessment Scores Knowledge Quiz 4.1 Knowledge Quiz 4.4 Knowledge Quiz 4.2 Knowledge Quiz 4.5 Knowledge Quiz 4.3 Notes: Geography Climate Zones and Biomes Pupil Workbook Year 3, Unit 4 Climate Zones and Biomes Knowledge Organiser Vocabulary Biome Definition The weather conditions in an area 1 Climate 8 Rainforest A thick forest that has a lot of rain over time A grassy plain in tropical and An area with similar plants and 9 Savannah 2 Biome subtropical regions with definition animals A waterless area with little or 10 Desert Smaller regions indicating where no vegetation 3 Vegetation belt vegetation grows An area that has mainly shrubs and 11 Chaparral Polar and Areas in the north and south of thorny bushes 4 subpolar zone the globe A large open area covered with 12 Grassland grass 5 Temperate zone Areas of mild temperature Deciduous A forest that has trees that lose 13 forest their leaves each year Equatorial and Area in the centre of the globe with 6 A forest made up of coniferous Tropical zones a hot temperature 14 Boreal forest plants in cold areas 7 Arid zone Areas north and south of the tropics 15 Tundra A flat, cold, treeless area Climate Equatorial zone Tropical zone Arid zone Mediterranean zone Temperate zone Subpolar zone Polar zone Biomes Polar Desert Tundra Boreal forest Tropical Rainforest Deciduous forest Grassland Savannah Desert Chaparral Challenges of a biome for humans Biomes in Europe Rainforest: Grassland • It can rain more than 250cm a -
Full Biological Resources Report Outline
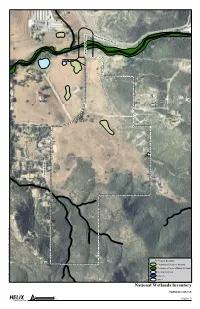
e H v ar i mo r n y G D r ove b Roa u d l C y r t n u o C Study Area e v i r D y e r d r o C F K - 4 1 / 2 2 / 7 0 1 0 - V O K d x m . I W N _ 5 g i F \ D J \ O I B \ p a M \ E S e v o r G y n Property Boundary o m r a H _ Freshwater Emergent Wetland 1 0 - V O Freshwater Forested/Shrub Wetland K \ V O K Freshwater Pond \ K \ S T Riverine C E J O R Other P \ : I National Wetlands Inventory HARMONY GROVE 0 350 N Feet Figure 5 e v i r D b k e e u r l C d o C i d o n y s c r E t n u o C Study Area e v i r D y e r d r o C F K - 4 1 / 2 2 / 7 0 Property Boundary 1 0 - V Mule Fat Scrub O K d x Southern Willow Riparian Forest m . n o i t a t Coast Live Oak Woodland e g e V _ 6 Diegan Coastal Sage Scrub g i F \ D J \ Diegan Coastal Sage Scrub - Disturbed O I B \ p a Coastal Sage-chaparral Scrub M \ E S e Mafic Southern Mixed Chaparral v o r G y n Granitic Southern Mixed Chaparral o m r a H _ Non-native Grassland 1 0 - V O Eucalyptus Woodland K \ V O K Non-native Vegetation \ K \ S T Disturbed Habitat C E J O R Developed P \ : I Vegetation HARMONY GROVE 0 350 N Feet Figure 6 the annual, introduced species that comprise the majority of species and biomass within the non-native grassland originated from the Mediterranean region, an area with a long history of agriculture and a climate similar to California. -

WWF Global 200 Reportfinal
habitats WWF Climate Change Campaign Climate change poses a serious threat to the survival of many species and to the well-being of people around the world. WWF’s campaign has three main aims: • to ensure that industrialised nations make substantial reductions in their domestic emissions of carbon dioxide—the main global warming gas— by 2010 • to promote the use of clean renewable energy in the developing world • to reduce the vulnerability of nature and economies to the impacts of climate change WWF Climate Change Campaign Director Jennifer Morgan c/o WWF US 1250 24th Street, NW Washington DC 20037 USA Tel: +1 202 822 3455 Fax: +1 202 331 2391 Website: www.panda.org/climate WWF’s mission is to stop the degradation of the planet’s natural environment and to build a future in which humans can live in harmony with nature, by: Global Warming • conserving the world’s biological diversity and Species Loss in • ensuring that the use of renewable resources is sustainable • promoting the reduction of pollution and wasteful consumption Globally Significant Terrestrial Ecosystems at risk www.davidsuzuki.org Jay R. Malcolm | Canran Liu | Laurie B. Miller | Tom Allnutt | Lara Hansen © 1986, WWF ® WWF Registered Trademark owner Published February 2002 by WWF-World Wide Fund for Nature (Formerly World Wildlife Fund), Gland, Switzerland. Any reproduction in full or in part of this publication must mention the title and credit the above-mentioned publisher as the copyright owner. © text 2002 WWF. All rights reserved. The material and the geographical designations in this report do not imply the expression of any opinion whatsoever on the part of WWF concerning the legal status of any country, territo- ry, or area, or concerning the delimitation of its frontiers or boundaries. -

Chaparral Fire Update Media & Fire Information (951) 940-6985
Chaparral Fire Update Media & Fire Information (951) 940-6985 Murrieta, Calif, August 30, 2021 — As of 6:00 pm on Sunday, August 29, 2021, California Interagency Incident Management Team 14 joined CAL FIRE and the Cleveland National Forest in Unified Command of the Chaparral Fire. Yesterday: During the day, crews continued to put in direct line in the south and east areas of the fire. Crews continued to insert into the wilderness with direct line and hose support. Thunderstorms and outflow winds east of the fire yesterday afternoon grounded air operations for a short time but did not affect firefighting operations. By the end of the day, fire retardant had been applied around the entire fire perimeter. Fire behavior overnight was moderate with crews continuing to construct and strengthen containment lines and suppress hotspots. Today: Planned work assignments include continuing hose lays to improve perimeter control, constructing direct handline when possible, mopping up a minimum of 100-200 feet interior to the fire control lines when possible, ensuring protection of all structures in the area of the fire. Fire Behavior: Live and dead vegetation—consisting of tall, dense chamise, manzanita, sugar bush, scrub oak and dry grass—is critically dry. There have been no recent fires or fuels treatments in the immediate fire area. Weather: Humidity is expected to increase in the low to middle elevations ahead of Hurricane Nora coming north from Baja. Higher elevations may continue to have lower relative humidity recoveries. Temperatures are predicted to be cooler in the next few days. Daytime heating and some instability may produce a few isolated afternoon thunderstorms. -

A Spatial Analysis Approach to the Global Delineation of Dryland Areas of Relevance to the CBD Programme of Work on Dry and Subhumid Lands
A spatial analysis approach to the global delineation of dryland areas of relevance to the CBD Programme of Work on Dry and Subhumid Lands Prepared by Levke Sörensen at the UNEP World Conservation Monitoring Centre Cambridge, UK January 2007 This report was prepared at the United Nations Environment Programme World Conservation Monitoring Centre (UNEP-WCMC). The lead author is Levke Sörensen, scholar of the Carlo Schmid Programme of the German Academic Exchange Service (DAAD). Acknowledgements This report benefited from major support from Peter Herkenrath, Lera Miles and Corinna Ravilious. UNEP-WCMC is also grateful for the contributions of and discussions with Jaime Webbe, Programme Officer, Dry and Subhumid Lands, at the CBD Secretariat. Disclaimer The contents of the map presented here do not necessarily reflect the views or policies of UNEP-WCMC or contributory organizations. The designations employed and the presentations do not imply the expression of any opinion whatsoever on the part of UNEP-WCMC or contributory organizations concerning the legal status of any country, territory or area or its authority, or concerning the delimitation of its frontiers or boundaries. 3 Table of contents Acknowledgements............................................................................................3 Disclaimer ...........................................................................................................3 List of tables, annexes and maps .....................................................................5 Abbreviations -

California's Diverse Vegetation Part 1
California’s Diverse Vegetation Part 1 GEO 351 Dr.Garver CA Flora and Fauna Diversity • Topography • Climates • Latitudinal range • Coastline • Human interaction - modified landscape in past few centuries Topography Topography & Climate Principal Biomes Principal Biomes Biome - a regional ecosystem characterized by distinct types of vegetation, animals, and microbes that have developed under specific soil and climatic conditions. Principal Biomes • Biomes - biogeographical units containing – Physical environments • climate • soils • landforms • water – 5 biomes in CA • Flora and fauna 5 Biomes • Coniferous Forests • Oak Woodlands • Grasslands & Marshlands • Desert Scrublands • Chaparral and Coastal Shrublands Floristic Provinces Floristic Provinces Floristic Provinces • geographic area with uniform composition of plant species. boundaries with adjacent provinces are soft, transitional areas where many species from both regions overlap. N. America – 13 floristic provinces – 4 in California: Californian, Vancouverian, Sonoran and Great Basin. CALIFORNIAN- Defined by its Mediterranean climate. Smallest in N. America, but greatest diversity of plants north of Mexico. Characteristic vegetation as chaparral/coastal sage scrub, oak woodland and grassland. These plants exhibit classic adaptations to California's hot dry summers and cool wet winters. VANCOUVERIAN - encompasses state's major forests and includes mixed evergreen and coniferous forests of pines and coast and sierran redwoods - transitional between Mediterranean climate vegetation -

Functional Diversity in the Hyper-Diverse Mangrove Communities in Papua New Guinea Lawong Balun [email protected]
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange Doctoral Dissertations Graduate School 12-2011 Functional Diversity in the Hyper-diverse Mangrove Communities in Papua New Guinea Lawong Balun [email protected] Recommended Citation Balun, Lawong, "Functional Diversity in the Hyper-diverse Mangrove Communities in Papua New Guinea. " PhD diss., University of Tennessee, 2011. https://trace.tennessee.edu/utk_graddiss/1166 This Dissertation is brought to you for free and open access by the Graduate School at Trace: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Doctoral Dissertations by an authorized administrator of Trace: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a dissertation written by Lawong Balun entitled "Functional Diversity in the Hyper-diverse Mangrove Communities in Papua New Guinea." I have examined the final electronic copy of this dissertation for form and content and recommend that it be accepted in partial fulfillment of the requirements for the degree of Doctor of Philosophy, with a major in Ecology and Evolutionary Biology. Taylor Feild, Major Professor We have read this dissertation and recommend its acceptance: Edward Shilling, Joe Williams, Stan Wulschleger Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official student records.) Functional Diversity in Hyper-diverse Mangrove Communities